Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into the DNA-binding mechanism of BCL11A: The integral role of ZnF6
Structure ( IF 4.4 ) Pub Date : 2024-10-17 , DOI: 10.1016/j.str.2024.09.022 Thibault Viennet, Maolu Yin, Abhilash Jayaraj, Woojin Kim, Zhen-Yu J. Sun, Yuko Fujiwara, Kevin Zhang, Davide Seruggia, Hyuk-Soo Seo, Sirano Dhe-Paganon, Stuart H. Orkin, Haribabu Arthanari
Structure ( IF 4.4 ) Pub Date : 2024-10-17 , DOI: 10.1016/j.str.2024.09.022 Thibault Viennet, Maolu Yin, Abhilash Jayaraj, Woojin Kim, Zhen-Yu J. Sun, Yuko Fujiwara, Kevin Zhang, Davide Seruggia, Hyuk-Soo Seo, Sirano Dhe-Paganon, Stuart H. Orkin, Haribabu Arthanari
|
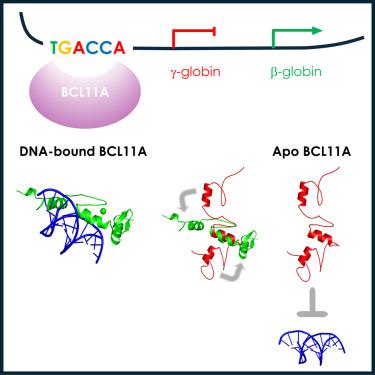
The transcription factor BCL11A is a critical regulator of the switch from fetal hemoglobin (HbF: α2γ2) to adult hemoglobin (HbA: α2β2) during development. BCL11A binds at a cognate recognition site (TGACCA) in the γ-globin gene promoter and represses its expression. DNA-binding is mediated by a triple zinc finger domain, designated ZnF456. Here, we report comprehensive investigation of ZnF456, leveraging X-ray crystallography and NMR to determine the structures in both the presence and absence of DNA. We delve into the dynamics and mode of interaction with DNA. Moreover, we discovered that the last zinc finger of BCL11A (ZnF6) plays a different role compared to ZnF4 and 5, providing a positive entropic contribution to DNA binding and γ-globin gene repression. Comprehending the DNA binding mechanism of BCL11A opens avenues for the strategic, structure-based design of novel therapeutics targeting sickle cell disease and β-thalassemia.
中文翻译:

BCL11A DNA 结合机制的结构见解:ZnF6 的不可或缺的作用
转录因子 BCL11A 是发育过程中从胎儿血红蛋白 (HbF: α2γ2) 转变为成人血红蛋白 (HbA: α2β2) 的关键调节因子。BCL11A 在 γ-珠蛋白基因启动子的同源识别位点 (TGACCA) 处结合并抑制其表达。DNA 结合由三重锌指结构域介导,称为 ZnF456。在这里,我们报告了对 ZnF456 的全面研究,利用 X 射线晶体学和 NMR 来确定 DNA 存在和不存在的结构。我们深入研究了与 DNA 相互作用的动力学和模式。此外,我们发现 BCL11A (ZnF6) 的最后一个锌指与 ZnF4 和 5 相比起着不同的作用,为 DNA 结合和 γ-珠蛋白基因抑制提供正熵贡献。了解 BCL11A 的 DNA 结合机制为针对镰状细胞病和 β-地中海贫血的新型疗法的战略性、基于结构的设计开辟了道路。
更新日期:2024-10-17
中文翻译:

BCL11A DNA 结合机制的结构见解:ZnF6 的不可或缺的作用
转录因子 BCL11A 是发育过程中从胎儿血红蛋白 (HbF: α2γ2) 转变为成人血红蛋白 (HbA: α2β2) 的关键调节因子。BCL11A 在 γ-珠蛋白基因启动子的同源识别位点 (TGACCA) 处结合并抑制其表达。DNA 结合由三重锌指结构域介导,称为 ZnF456。在这里,我们报告了对 ZnF456 的全面研究,利用 X 射线晶体学和 NMR 来确定 DNA 存在和不存在的结构。我们深入研究了与 DNA 相互作用的动力学和模式。此外,我们发现 BCL11A (ZnF6) 的最后一个锌指与 ZnF4 和 5 相比起着不同的作用,为 DNA 结合和 γ-珠蛋白基因抑制提供正熵贡献。了解 BCL11A 的 DNA 结合机制为针对镰状细胞病和 β-地中海贫血的新型疗法的战略性、基于结构的设计开辟了道路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号