Diabetologia ( IF 8.4 ) Pub Date : 2024-10-15 , DOI: 10.1007/s00125-024-06286-2 Miwon Ahn, Sangeeta Dhawan, Erika M. McCown, Pablo A. Garcia, Supriyo Bhattacharya, Roland Stein, Debbie C. Thurmond
|
Aims/hypothesis
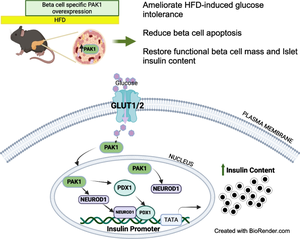
p21 (CDC42/RAC1) activated kinase 1 (PAK1) is depleted in type 2 diabetic human islets compared with non-diabetic human islets, and acute PAK1 restoration in the islets can restore insulin secretory function ex vivo. We hypothesised that beta cell-specific PAK1 enrichment in vivo can mitigate high-fat-diet (HFD)-induced glucose intolerance by increasing the functional beta cell mass.
Methods
Human islets expressing exogenous PAK1 specifically in beta cells were used for bulk RNA-seq. Human EndoC-βH1 cells overexpressing myc-tagged PAK1 were used for chromatin immunoprecipitation (ChIP) and ChIP-sequencing (ChIP-seq). Novel doxycycline-inducible beta cell-specific PAK1-expressing (iβPAK1-Tg) mice were fed a 45% HFD pre-induction for 3 weeks and for a further 3 weeks with or without doxycycline induction. These HFD-fed mice were evaluated for GTT, ITT, 6 h fasting plasma insulin and blood glucose, body composition, islet insulin content and apoptosis.
Results
Beta cell-specific PAK1 enrichment in type 2 diabetes human islets resulted in decreased beta cell apoptosis and increased insulin content. RNA-seq showed an upregulation of INS gene transcription by PAK1. Using clonal human beta cells, we found that PAK1 protein was localised in the cytoplasm and the nucleus. ChIP studies revealed that nuclear PAK1 enhanced pancreatic and duodenal homeobox1 (PDX1) and neuronal differentiation 1 (NEUROD1) binding to the INS promoter in a glucose-responsive manner. Importantly, the iβPAK1-Tg mice, when challenged with HFD and doxycycline induction displayed enhanced glucose tolerance, increased islet insulin content and reduced beta cell apoptosis when compared with iβPAK1-Tg mice without doxycycline induction.
Conclusions/interpretation
PAK1 plays an unforeseen and beneficial role in beta cells by promoting insulin biogenesis via enhancing the expression of PDX1, NEUROD1 and INS, along with anti-apoptotic effects, that culminate in increased insulin content and beta cell mass in vivo and ameliorate diet-induced glucose intolerance.
Data availability
The raw and processed RNA-seq data and ChIP-seq data, which has been made publicly available at Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/, can be accessed in GSE239382.
Graphical Abstract
中文翻译:

β 细胞特异性 PAK1 富集通过促进胰岛素生物发生和最大限度地减少 β 细胞凋亡来改善饮食诱导的小鼠葡萄糖耐受不良
目标/假设
与非糖尿病人胰岛相比,2 型糖尿病人胰岛中 p21 (CDC42/RAC1) 活化激酶 1 (PAK1) 耗尽,胰岛中急性 PAK1 恢复可以体外恢复胰岛素分泌功能。我们假设体内 β 细胞特异性 PAK1 富集可以通过增加功能性 β 细胞质量来缓解高脂肪饮食 (HFD) 诱导的葡萄糖耐受不良。
方法
在 β 细胞中特异性表达外源 PAK1 的人胰岛用于大量 RNA-seq。过表达 myc 标记的 PAK1 的人 EndoC-βH1 细胞用于染色质免疫沉淀 (ChIP) 和 ChIP-测序 (ChIP-seq)。新型多西环素诱导的 β 细胞特异性 PAK1 表达 (iβPAK1-Tg) 小鼠在诱导前喂食 45% HFD,持续 3 周,再喂食 3 周,有或没有多西环素诱导。评估这些 HFD 喂养的小鼠的 GTT 、 ITT 、 6 h 空腹血浆胰岛素和血糖、体成分、胰岛胰岛素含量和细胞凋亡。
结果
2 型糖尿病人胰岛中 β 细胞特异性 PAK1 富集导致 β 细胞凋亡减少和胰岛素含量增加。RNA-seq 显示 PAK1 上调 INS 基因转录。使用克隆人 β 细胞,我们发现 PAK1 蛋白位于细胞质和细胞核中。ChIP 研究表明,核 PAK1 以葡萄糖反应方式增强胰腺和十二指肠同源盒 1 (PDX1) 和神经元分化 1 (NEUROD1) 与 INS 启动子的结合。重要的是,与未诱导多西环素的 iβPAK1-Tg 小鼠相比,当接受 HFD 和多西环素诱导攻击时,iβPAK1-Tg 小鼠表现出葡萄糖耐量增强、胰岛胰岛素含量增加和 β 细胞凋亡减少。
结论/解释
PAK1 通过增强 PDX1、NEUROD1 和 INS 的表达促进胰岛素生物发生,以及抗凋亡作用,最终增加体内胰岛素含量和 β 细胞质量,并改善饮食诱导的葡萄糖耐受,从而在 β 细胞中发挥意想不到的有益作用。
数据可用性
原始和处理的 RNA-seq 数据和 ChIP-seq 数据已在 https://www.ncbi.nlm.nih.gov/geo/ 的基因表达综合 (GEO) 上公开提供,可以在 GSE239382 中访问。































 京公网安备 11010802027423号
京公网安备 11010802027423号