Arthritis & Rheumatology ( IF 11.4 ) Pub Date : 2024-10-14 , DOI: 10.1002/art.43019 Mariana J. Kaplan
|
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune syndrome characterized by a dysregulated immune response, leading to widespread inflammation and tissue damage. In recent years, research has highlighted the involvement of neutrophil extracellular traps (NETs) in the pathogenesis of lupus.1 This case study aims to illustrate the clinical manifestations of lupus and explore the role of NETs in disease pathogenesis, diagnosis, and treatment.
Case presentation
The patient, a 31-year-old woman, presents to the rheumatology clinic with a 6-month history of fatigue, joint pain, intermittent fevers, and facial rash exacerbated by sun exposure. These symptoms were first noticed after the resolution of an upper respiratory tract infection. She also reports experiencing nonpainful recurrent oral ulcers, Raynaud phenomenon, and hair loss over the past 3 months. She has taken acetaminophen for the joint pain and has tried to stay away from the sun, but her symptoms have persisted. On physical examination, she displays an erythematous macular malar rash that spares the nasolabial folds, oral ulcers, and tenderness and swelling in multiple joints in hands and feet as well as in her knees. There is a maculopapular erythematous rash in her upper arms. Laboratory tests reveal leukopenia, lymphopenia, mild neutropenia, elevated erythrocyte sedimentation rate, normal C-reactive protein levels, increased levels of antinuclear antibodies (homogenous pattern), anti–double-stranded DNA (anti-dsDNA) and anti-Ro antibodies, and decreased C3 and C4 levels. Her renal function test results are normal and there is no proteinuria. The lipoprotein profile reveals low high-density lipoprotein (HDL) and elevated low-density lipoprotein and total cholesterol. A carotid ultrasound scan, done for research purposes as part of a lupus cardiovascular cohort assessment, reveals a significant increase in carotid intimal media thickness bilaterally. An evaluation of HDL function reveals impaired cholesterol efflux capacity. A plasma sample sent to a research laboratory reveals elevated myeloperoxidase (MPO):DNA remnants and citrullinated histone H3:DNA remnants consistent with NETs, and a flow cytometry analysis of peripheral blood mononuclear cells reveals the presence of low-density granulocytes (LDGs).
Given the clinical presentation and laboratory findings, the patient would be diagnosed as having SLE, preclinical atherosclerosis, and dyslipidemia and started on an antimalarial, a statin, and low-dose prednisone. The patient would also be advised regarding sun protection, vitamin D intake, warranted vaccinations, and other general preventive measures for SLE comorbidities.
SLE, neutrophil dysregulation, and NETs
The patient's case illustrates the classic presentation of SLE, characterized by a constellation of constitutional symptoms, mucocutaneous manifestations, and multisystem involvement, in association with evidence of cardiometabolic dysfunction. The pathogenesis of SLE involves a complex interplay between innate and adaptive immune responses. Although neutrophils have been proposed to play fundamental roles in the pathogenesis of SLE, including previous descriptions of aberrant clearance of apoptotic neutrophils and the LE cell phenomenon, recent discoveries over the past 15 years have highlighted and identified novel mechanisms by which these cells may play fundamental roles in the initiation and perpetuation of a variety of systemic autoimmune diseases, including SLE, antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid arthritis, and idiopathic inflammatory myopathies.2-5
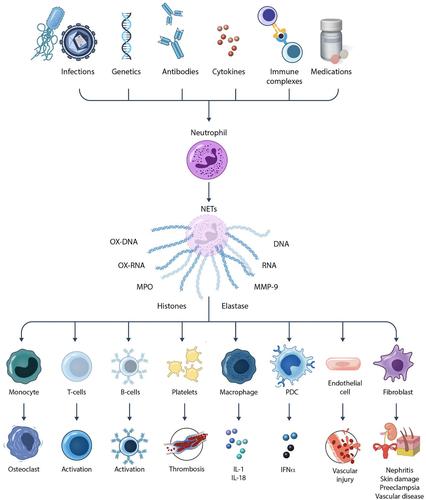
One of the neutrophil features that has been identified by various research groups as becoming dysregulated in autoimmunity is the formation of NETs.6 These are web-like structures composed of DNA, RNA, histones, and various antimicrobial proteins, which are released to the extracellular space by activated neutrophils in response to a variety of microbial and sterile inflammatory stimuli. In SLE, dysregulated NET formation and impaired clearance of these structures have been reported to lead to their accumulation within tissues and to their extended half-life in circulation, potentially contributing to perpetuating autoantigen generation and modification, inflammation, and tissue damage.6, 7 NETs may contribute to the pathogenesis of lupus nephritis, lupus-associated skin disease, thrombosis, vascular damage, and tissue injury in various organs, including pregnancy complications such as preeclampsia.6, 8, 9
The aberrant exposure within NETs of modified autoantigens, including oxidized nucleic acids and posttranslationally modified histones,10 may contribute to triggering the production of autoantibodies, including anti-dsDNA and antinucleosome antibodies, perpetuating the autoimmune responses in SLE. Furthermore, NETs promote the in vitro activation of plasmacytoid dendritic cells (DCs) and the production of type I interferons (IFNs), driving systemic inflammation and amplifying immune dysregulation in this disease.6
Mechanism of NET formation and triggers in SLE
NETosis is a unique form of cell death distinct from apoptosis and necrosis. It involves the release of neutrophil chromatin bound with various antimicrobial proteins, such as MPO and neutrophil elastase. NET formation can occur via several pathways. The classic pathway involves reactive oxygen species (ROS) generation by the NADPH oxidase pathway. This leads to the activation of protein-arginine deiminase 4 (PAD4), which catalyzes the conversion of arginine residues to citrulline in histones, resulting in chromatin decondensation. Subsequently, the nuclear envelope and granule membranes disintegrate, allowing the release of chromatin fibers decorated with antimicrobial proteins. The presence of citrullinated histones is a hallmark of NETs, distinguishing them from other extracellular DNA structures (reviewed in Wigerblad and Kaplan1).
In the context of SLE, several factors contribute to the enhanced formation and defective clearance of NETs. These include oxidative stress, autoantibodies, immune complexes, autoimmunity-inducing drugs, and proinflammatory cytokines.10 Various genetic polymorphisms that confer risk for SLE, including IRF-5, STAT-4, and PTPN22, are also associated to enhanced NET formation.11, 12 As mentioned above, ROS play a crucial role in NET formation, and patients with lupus exhibit elevated levels of oxidative stress, which correlates with increased NETosis. Furthermore, lupus autoantibodies, particularly those targeting nuclear antigens, such as anti-RNP immune complexes, can directly induce NET formation.3 These autoantibodies and immune complexes bind to neutrophil surface receptors, triggering intracellular signaling cascades that lead to NET release.
A putative link between infections and lupus flares has been reported.13 It is possible that innate immune activation following some infections, including the formation of NETs, could link microbial exposure to disease flares. Furthermore, UV light (also linked with disease flares), has been associated with the formation of NETs.14 As such, factors well-known to be associated with exacerbations of lupus symptoms may do so, in part, by inducing neutrophil dysregulation leading to NET formation and extrusion of autoantigens, alarmins, and other signals associated with innate and adaptive immune activation.
In the case of this patient, she had noticed that her symptoms had appeared and were exacerbated during sun exposure and following an upper respiratory tract infection. One can posit that these triggers may have promoted flares, at least in part, through the induction of NETs in skin, mucosal sites, and other organs. Furthermore, the mild neutropenia observed in this patient may be secondary to enhanced NET formation leading to neutrophil cell death; this is supported by the finding of elevated NET remnants in her plasma.
LDGs in SLE
LDGs have garnered significant attention in the context of SLE because of their distinctive characteristics and potential implications in disease pathogenesis. These cells, which are found in circulation at increased levels in a significant proportion of patients SLE and are typically purified from the mononuclear cell layer after density centrifugation (hence, their name because of having lower density than regular neutrophils), exhibit unique phenotypic and functional properties when compared with their higher-density counterparts.15 These properties include altered cytokine production, enhanced ability to form NETs in the absence of added stimulation, exacerbated ability to synthesize ROS, and enhanced ability to damage the endothelium through matrix-metalloproteinases.6, 15 The increased prevalence of LDGs in patients with SLE, coupled with their potential to contribute to disease pathology through the release of inflammatory mediators and autoantigens, makes them a subject of interest for understanding disease mechanisms and progression in SLE.
Clinically, LDGs have emerged as potential biomarkers for SLE owing to their association with disease activity and flares.16, 17 Elevated levels of LDGs have been observed in patients with SLE during active disease phases, and their counts correlate with disease severity, coronary plaque severity, and vascular wall inflammation, as assessed by coronary computed tomography and fluorodeoxyglucose positron emission tomography, respectively.16, 17 Furthermore, LDGs have been associated with impaired lipoprotein (HDL) function, because they promote a loss of its atheroprotective function through oxidation during NET formation.18 As such, measuring LDG levels could provide valuable insights into disease activity, helping clinicians to monitor and potentially predict flares or response to therapy. These studies require large cohorts and further validation before deciding whether LDGs add additional value in the management of SLE. The identification of specific markers or functional characteristics of LDGs could facilitate more targeted therapeutic approaches, aiming to modulate the inflammatory responses mediated by these cells. Monitoring LDG levels could therefore provide insights into ongoing vascular damage and the efficacy of therapeutic interventions aimed at reducing inflammation and protecting vascular health.
In the case of this patient, there was evidence of accelerated vascular damage as assessed by carotid intima-media thickness, as well as dysregulated lipoprotein panel with impaired HDL function, elevated LDGs, and NET remnants in circulation. It is possible that these abnormalities were triggered, at least in part, by LDGs and associated NET formation, although such measurements were not readily available.
NETs and autoantigen exposure
One of the central pathogenic features of lupus is the generation of autoantibodies against nuclear components such as DNA, histones, and other chromatin-associated proteins. NETs expose these nuclear antigens to the immune system in an immunogenic context. During NET formation, neutrophils release various nuclear proteins, including modified histones, high-mobility group box 1, LL37, which can act as autoantigens, and DAMPs6 (reviewed in Wigerblad and Kaplan1). The release of these nuclear proteins in the form of NETs provides a rich source of modified autoantigens that can stimulate the production of autoantibodies. This process is further amplified by the presence of DCs, macrophages, nonprofessional antigen-presenting cells, and various stromal cells that can take up NET components, process them, and present them to autoreactive T and B cells.19, 20 This phenomenon leads to a vicious cycle of autoantigen exposure and autoantibody production, perpetuating the autoimmune response. In addition, NET formation leads to enhanced oxidation of nucleic acids (genomic and mitochondrial DNA and RNA). The oxidation of nucleic acids enhances the half-life of these structures by making their degradation by nucleases more cumbersome. This increases the sensing by intracellular sensors of nucleic acids, including the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) and endosomal toll-like receptor pathways, promoting the synthesis of type I IFN responses, a hallmark of lupus pathogenesis.10, 21
Impaired NET clearance
In healthy individuals, NETs are rapidly cleared by nucleases such as DNases, which degrade the extracellular DNA. However, patients with lupus often exhibit defects in NET clearance. This impaired clearance can be attributed to several factors, including the above-mentioned nucleic acid oxidation, genetically driven reduced DNase activity, presence of DNase inhibitors, and other factors that contribute to the formation of DNase-resistant NETs. Persistent NETs in the circulation or those deposited in tissues can serve as a continuous source of autoantigens and proinflammatory stimuli.7, 10 Various studies have shown that a significant proportion of patients with lupus have elevated levels of circulating NETs and that these NETs are often resistant to degradation.
NETs and inflammation
NETs are not only a source of autoantigens but also potent inducers of inflammation (Figure 1). The components of NETs, including histones, proteases, and antimicrobial peptides, can act as DAMPs that trigger the release of proinflammatory cytokines and chemokines. These inflammatory mediators recruit and activate other immune cells, amplifying the inflammatory response.

In lupus, the chronic presence of NETs can lead to sustained inflammation in various tissues, including the skin, kidneys, placenta, and blood vessels.6, 8 This persistent inflammation is a hallmark of lupus and may contribute to the clinical manifestations of the disease, such as nephritis, vasculitis, and skin rash. Moreover, the inflammatory milieu created and exacerbated by NETs can promote further NETosis, creating a feedback loop that exacerbates tissue damage and autoimmunity.
NETs and organ damage
The deposition of NETs in tissues is associated with organ damage in lupus. In the kidneys, for example, NETs can accumulate in the glomeruli and tubular-interstitial compartment, contributing to the pathogenesis of lupus glomerulonephritis, a common and severe manifestation of SLE.6 The presence of NETs in the kidneys can induce local inflammation and complement fibroblast activation and endothelial cell damage, contributing to the pathology of lupus nephritis.22 NETs have also been found in lupus skin, where they are detected in areas of clinical disease.6 It has been posited that this local NET production may contribute to activating other cells in the skin, as well as to local type I IFN synthesis. In turn, type I IFNs may prime neutrophils to synthesize more NETs.3
Similarly, in blood vessels, NETs can contribute to the development of vasculitis and atherosclerosis. NETs can activate and damage endothelial cells and promote the recruitment of inflammatory cells to the vessel wall.3, 23 This NET-mediated process can lead to endothelial dysfunction, increased vascular permeability, and thrombosis. Patients with lupus have a higher risk of cardiovascular disease, and NETs are believed to play a role in this increased risk by promoting vascular inflammation and injury.24
This patient had evidence of increased carotid intimal media thickness at a young age, which is unusual and likely related to vascular damage associated with SLE. She also has evidence of dyslipidemia with aberrant HDL levels and function. These abnormalities have been previously linked to dysregulated NET formation and to increased LDG levels in circulation. As such, it is possible that neutrophil dysregulation has played a prominent role in vascular damage, which has likely preceded SLE diagnosis.
NETs as biomarkers
Measurement of circulating NET and LDG markers may aid in the diagnosis and monitoring of disease activity in patients with lupus, providing valuable insights into disease progression and treatment response. NETs can be detected in blood and tissue samples using various techniques, such as immunofluorescence microscopy, enzyme-linked immunosorbent assay, and semiautomated live-cell analysis.10 Markers used to identify NETs include citrullinated histone:DNA complexes, DNA-MPO or DNA-neutrophil and elastase complexes.25 The use of cell-free DNA is not a specific marker of NET formation and should be avoided as a sole marker. Several studies have demonstrated a correlation among elevated NET levels, LDGs, and increased disease activity in patients with SLE.17, 24 High levels of NETs are associated with active disease, renal involvement, and increased risk of flares. It is possible that, once properly validated, monitoring NET levels could provide valuable information for assessing disease progression and treatment response. It is possible that NETs could be used to identify patients at risk of relapse and guide preemptive therapeutic interventions. Furthermore, anti-NET antibodies targeting NET components, such as citrullinated histones and MPO, have emerged as potential biomarkers for lupus diagnosis and stratification but require further validation. Finally, previous studies have linked levels of LDGs to vascular damage as mentioned above.
Although NETs and LDGs are not currently used as clinical biomarkers of vascular risk, it is possible that they will be incorporated in the future in the clinical decision-making of patients with SLE. In the case of this patient, her active disease was associated with the presence of elevated LDGs and NETs. It is possible that these NETs have contributed to her active skin and joint disease, although tissue biopsies would have been needed to document their involvement.
Therapeutic implications
Understanding the role of NETs in the pathogenesis of lupus opens new therapeutic avenues. Targeting NET formation, promoting NET clearance, or neutralizing NET components could potentially mitigate the harmful effects of NETs in lupus. Several strategies are being explored in this regard. Current therapies for SLE include immunosuppressive drugs, such as corticosteroids, antimalarials, and biologics targeting B cells and cytokines. Some of these therapies, such as hydroxychloroquine, JAK inhibitors (tofacitinib),12 some anti–B cell therapies,26 and the type I IFN receptor blocker anifrolumab,24 have been reported to reduce NET formation and/or LDG numbers and the deleterious effects of NETs. However, there is a need for more targeted therapies specifically designed to inhibit NETosis and LDGs, to suppress their deleterious effects in a more specific manner (reviewed in Wigerblad and Kaplan1).
Several potential therapeutic agents targeting NETs are under investigation, including the following. (1) PAD4 inhibitors, which can prevent histone citrullination and NET formation, but their clinical applicability remains to be determined. (2) NADPH oxidase inhibitors and/or inhibitors of mitochondrial ROS, which can reduce ROS production and inhibit NETosis; however, clinical strategies for this pathway remain to be systematically studied. Of note, the antioxidant N-acetyl-cysteine, a compound that can inhibit NET formation in vitro, has shown potential efficacy in SLE.27, 28 (3) DNase therapy, specifically with DNase I and DNase1L3, can degrade DNA in NETs, reducing their proinflammatory effects.29 (4) Anti-IFN therapy targeting type I IFNs can reduce NET-induced inflammation and autoimmunity, and the role of this approach in inhibiting vascular disease in SLE is currently being tested (NCT05440422).
In the patient's case, antimalarial use may have multiprong beneficial effects, including resolution/control of clinical symptoms and cardiovascular prevention and direct effects on the dysregulation of NET formation. Whether adding other drugs or biologics will further prevent damage mediated by innate immune dysregulation will have to be determined by the patient's response to treatment.
Future research should focus on identifying additional targets for NET inhibition and developing combination therapies to enhance treatment efficacy. Overall, accumulating evidence indicates that NETs play a significant role in the pathogenesis of SLE by promoting autoimmunity and inflammation. It remains to be further characterized whether they serve as valuable biomarkers for disease activity and flares and whether targeting NET formation may be a promising therapeutic strategy. In this clinical case, it is possible that in the future, measurements of NETs and LDGs will add clinical information regarding diagnosis, prognosis, and disease severity and contribute to further understanding mechanisms of flares following infections and UV light exposure.
Conclusions
This case study underscores the critical role of NETs in the pathogenesis of SLE and highlights the clinical implications of NET biomarkers in disease diagnosis and management. By elucidating the mechanisms underlying NET-mediated inflammation, clinicians can develop targeted therapies to mitigate disease activity and improve outcomes in patients with lupus. Further research is needed to validate the diagnostic and therapeutic utility of NET-targeted interventions and optimize their integration into clinical practice.
AUTHOR CONTRIBUTIONS
Dr Kaplan contributed to at least one of the following manuscript preparation roles: conceptualization AND/OR methodology, software, investigation, formal analysis, data curation, visualization, and validation AND drafting or reviewing/editing the final draft. As corresponding author, Dr Kaplan provided the final approval of the version to be published, and takes responsibility for the affirmations regarding article submission (eg, not under consideration by another journal), the integrity of the data presented, and the statements regarding compliance with institutional review board/Helsinki Declaration requirements.
Supporting Information
REFERENCES
中文翻译:

探索中性粒细胞胞外陷阱 (NET) 在 SLE 中的作用:临床案例研究和综合综述
点击文章标题阅读更多内容。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号