当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An enzymatic cascade for high-yield and stereoselective synthesis of 4-fluoro-L-threonine
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-15 , DOI: 10.1016/j.checat.2024.101148 Alberto De Maria, Manuel Nieto-Domínguez, Phillip T. Lowe, David O′Hagan, Pablo I. Nikel
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-15 , DOI: 10.1016/j.checat.2024.101148 Alberto De Maria, Manuel Nieto-Domínguez, Phillip T. Lowe, David O′Hagan, Pablo I. Nikel
|
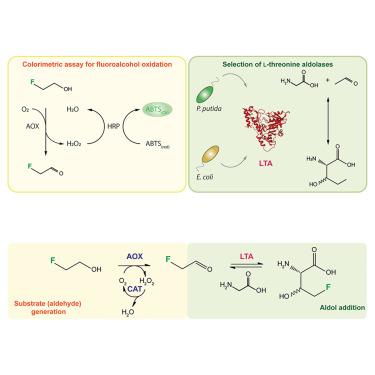
The critical role of fluorine in bioactive molecule design requires selective fluorination methods for synthesizing novel building blocks, such as fluorinated amino acids. Here, we focused on L-threonine aldolases (LTAs), enzymes that mediate reversible aldol additions to the α carbon of glycine. Their C–C bond formation ability and substrate flexibility make these enzymes ideal catalysts for fluorine biocatalysis. We harnessed the promiscuous activity of the LTAs isolated from either Escherichia coli or Pseudomonas putida on 2-fluoroacetaldehyde in a two-step enzymatic cascade for efficient 4-fluoro-L-threonine synthesis. By implementing 2-fluoroethanol as the primary fluorodonor in these cascades, we demonstrated that the LTA enzyme isolated from P . putida mediates a high 4-fluoro-L-threonine yield (>90%) while displaying stereoselectivity for the L-syn form.
中文翻译:

用于 4-氟-L-苏氨酸的高产率和立体选择性合成的酶联反应
氟在生物活性分子设计中的关键作用需要选择性氟化方法来合成新型结构单元,例如氟化氨基酸。在这里,我们专注于 L-苏氨酸醛缩酶 (LTA),这是一种介导可逆羟醛添加到甘氨酸α碳中的酶。它们的 C-C 键形成能力和底物柔韧性使这些酶成为氟生物催化的理想催化剂。我们利用从大肠杆菌或恶臭假单胞菌中分离的 LTA 对 2-氟乙醛的混杂活性,以两步酶联反应实现高效的 4-氟-L-苏氨酸合成。通过实施 2-氟乙醇作为这些级联反应中的主要氟供体,我们证明从恶臭假单胞菌中分离的 LTA 酶介导高 4-氟-L-苏氨酸产量 (>90%),同时对 L-syn 形式表现出立体选择性。
更新日期:2024-10-15
中文翻译:

用于 4-氟-L-苏氨酸的高产率和立体选择性合成的酶联反应
氟在生物活性分子设计中的关键作用需要选择性氟化方法来合成新型结构单元,例如氟化氨基酸。在这里,我们专注于 L-苏氨酸醛缩酶 (LTA),这是一种介导可逆羟醛添加到甘氨酸α碳中的酶。它们的 C-C 键形成能力和底物柔韧性使这些酶成为氟生物催化的理想催化剂。我们利用从大肠杆菌或恶臭假单胞菌中分离的 LTA 对 2-氟乙醛的混杂活性,以两步酶联反应实现高效的 4-氟-L-苏氨酸合成。通过实施 2-氟乙醇作为这些级联反应中的主要氟供体,我们证明从恶臭假单胞菌中分离的 LTA 酶介导高 4-氟-L-苏氨酸产量 (>90%),同时对 L-syn 形式表现出立体选择性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号