Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Retinoic acid and TGF-β orchestrate organ-specific programs of tissue residency
Immunity ( IF 25.5 ) Pub Date : 2024-10-14 , DOI: 10.1016/j.immuni.2024.09.015 Andreas Obers, Tobias Poch, Grace Rodrigues, Susan N. Christo, Luke C. Gandolfo, Raissa Fonseca, Ali Zaid, Joey En Yu Kuai, Hongjin Lai, Pirooz Zareie, Marina H. Yakou, Lachlan Dryburgh, Thomas N. Burn, James Dosser, Frank A. Buquicchio, Caleb A. Lareau, Calum Walsh, Louise Judd, Maria Rafailia Theodorou, Katharina Gutbrod, Peter Dörmann, Jenny Kingham, Tim Stinear, Axel Kallies, Jan Schroeder, Scott N. Mueller, Simone L. Park, Terence P. Speed, Ansuman T. Satpathy, Tri Giang Phan, Christoph Wilhelm, Colby Zaph, Maximilien Evrard, Laura K. Mackay
Immunity ( IF 25.5 ) Pub Date : 2024-10-14 , DOI: 10.1016/j.immuni.2024.09.015 Andreas Obers, Tobias Poch, Grace Rodrigues, Susan N. Christo, Luke C. Gandolfo, Raissa Fonseca, Ali Zaid, Joey En Yu Kuai, Hongjin Lai, Pirooz Zareie, Marina H. Yakou, Lachlan Dryburgh, Thomas N. Burn, James Dosser, Frank A. Buquicchio, Caleb A. Lareau, Calum Walsh, Louise Judd, Maria Rafailia Theodorou, Katharina Gutbrod, Peter Dörmann, Jenny Kingham, Tim Stinear, Axel Kallies, Jan Schroeder, Scott N. Mueller, Simone L. Park, Terence P. Speed, Ansuman T. Satpathy, Tri Giang Phan, Christoph Wilhelm, Colby Zaph, Maximilien Evrard, Laura K. Mackay
|
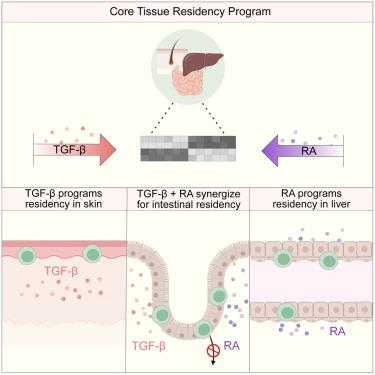
Tissue-resident memory T (TRM ) cells are integral to tissue immunity, persisting in diverse anatomical sites where they adhere to a common transcriptional framework. How these cells integrate distinct local cues to adopt the common TRM cell fate remains poorly understood. Here, we show that whereas skin TRM cells strictly require transforming growth factor β (TGF-β) for tissue residency, those in other locations utilize the metabolite retinoic acid (RA) to drive an alternative differentiation pathway, directing a TGF-β-independent tissue residency program in the liver and synergizing with TGF-β to drive TRM cells in the small intestine. We found that RA was required for the long-term maintenance of intestinal TRM populations, in part by impeding their retrograde migration. Moreover, enhanced RA signaling modulated TRM cell phenotype and function, a phenomenon mirrored in mice with increased microbial diversity. Together, our findings reveal RA as a fundamental component of the host-environment interaction that directs immunosurveillance in tissues.
中文翻译:

视黄酸和 TGF-β 协调器官特异性组织驻留程序
组织驻留记忆 T (TRM) 细胞是组织免疫不可或缺的一部分,它们存在于不同的解剖部位,在那里它们粘附于一个共同的转录框架。这些细胞如何整合不同的局部线索以适应共同的 TRM 细胞命运仍然知之甚少。在这里,我们表明,虽然皮肤 TRM 细胞严格需要转化生长因子 β (TGF-β) 才能进行组织驻留,但其他位置的细胞利用代谢物视黄酸 (RA) 来驱动另一种分化途径,指导肝脏中 TGF β非依赖性组织驻留程序,并与 TGF-β协同驱动小肠中的 TRM 细胞。我们发现 RA 是肠道 TRM 群体长期维持所必需的,部分原因是通过阻碍它们的逆行迁移。此外,增强的 RA 信号传导调节了 TRM 细胞表型和功能,这种现象在微生物多样性增加的小鼠中得到了反映。总之,我们的研究结果揭示了 RA 是指导组织中免疫监视的宿主-环境相互作用的基本组成部分。
更新日期:2024-10-14
中文翻译:

视黄酸和 TGF-β 协调器官特异性组织驻留程序
组织驻留记忆 T (TRM) 细胞是组织免疫不可或缺的一部分,它们存在于不同的解剖部位,在那里它们粘附于一个共同的转录框架。这些细胞如何整合不同的局部线索以适应共同的 TRM 细胞命运仍然知之甚少。在这里,我们表明,虽然皮肤 TRM 细胞严格需要转化生长因子 β (TGF-β) 才能进行组织驻留,但其他位置的细胞利用代谢物视黄酸 (RA) 来驱动另一种分化途径,指导肝脏中 TGF β非依赖性组织驻留程序,并与 TGF-β协同驱动小肠中的 TRM 细胞。我们发现 RA 是肠道 TRM 群体长期维持所必需的,部分原因是通过阻碍它们的逆行迁移。此外,增强的 RA 信号传导调节了 TRM 细胞表型和功能,这种现象在微生物多样性增加的小鼠中得到了反映。总之,我们的研究结果揭示了 RA 是指导组织中免疫监视的宿主-环境相互作用的基本组成部分。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号