Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CRISPR screens unveil nutrient-dependent lysosomal and mitochondrial nodes impacting intestinal tissue-resident memory CD8+ T cell formation
Immunity ( IF 25.5 ) Pub Date : 2024-10-14 , DOI: 10.1016/j.immuni.2024.09.013 Jana L. Raynor, Nicholas Collins, Hao Shi, Cliff Guy, Jordy Saravia, Seon Ah Lim, Nicole M. Chapman, Peipei Zhou, Yan Wang, Yu Sun, Isabel Risch, Haoran Hu, Anil KC, Renqiang Sun, Sharad Shrestha, Hongling Huang, Jon P. Connelly, Shondra M. Pruett-Miller, Miguel Reina-Campos, Ananda W. Goldrath, Yasmine Belkaid, Hongbo Chi
Immunity ( IF 25.5 ) Pub Date : 2024-10-14 , DOI: 10.1016/j.immuni.2024.09.013 Jana L. Raynor, Nicholas Collins, Hao Shi, Cliff Guy, Jordy Saravia, Seon Ah Lim, Nicole M. Chapman, Peipei Zhou, Yan Wang, Yu Sun, Isabel Risch, Haoran Hu, Anil KC, Renqiang Sun, Sharad Shrestha, Hongling Huang, Jon P. Connelly, Shondra M. Pruett-Miller, Miguel Reina-Campos, Ananda W. Goldrath, Yasmine Belkaid, Hongbo Chi
|
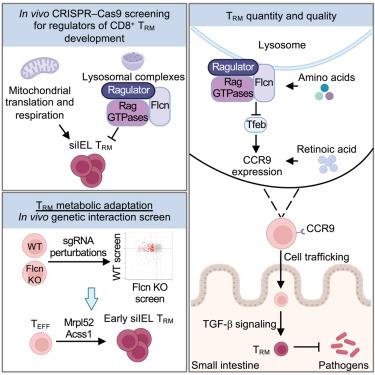
Nutrient availability and organelle biology direct tissue homeostasis and cell fate, but how these processes orchestrate tissue immunity remains poorly defined. Here, using in vivo CRISPR-Cas9 screens, we uncovered organelle signaling and metabolic processes shaping CD8+ tissue-resident memory T (TRM ) cell development. TRM cells depended on mitochondrial translation and respiration. Conversely, three nutrient-dependent lysosomal signaling nodes—Flcn, Ragulator, and Rag GTPases—inhibited intestinal TRM cell formation. Depleting these molecules or amino acids activated the transcription factor Tfeb, thereby linking nutrient stress to TRM programming. Further, Flcn deficiency promoted protective TRM cell responses in the small intestine. Mechanistically, the Flcn-Tfeb axis restrained retinoic acid-induced CCR9 expression for migration and transforming growth factor β (TGF-β)-mediated programming for lineage differentiation. Genetic interaction screening revealed that the mitochondrial protein Mrpl52 enabled early TRM cell formation, while Acss1 controlled TRM cell development under Flcn deficiency-associated lysosomal dysregulation. Thus, the interplay between nutrients, organelle signaling, and metabolic adaptation dictates tissue immunity.
中文翻译:

CRISPR 筛选揭示了影响肠道组织驻留记忆 CD8+ T 细胞形成的营养依赖性溶酶体和线粒体节点
营养可用性和细胞器生物学指导组织稳态和细胞命运,但这些过程如何协调组织免疫仍然不明确。在这里,使用 体内 CRISPR-Cas9 筛选,我们揭示了塑造 CD8+ 组织驻留记忆 T (TRM) 细胞发育的细胞器信号传导和代谢过程。TRM 细胞依赖于线粒体翻译和呼吸。相反,三个营养依赖性溶酶体信号节点 — Flcn、Rajurator 和 Rag GTP 酶 — 抑制肠道 TRM 细胞形成。消耗这些分子或氨基酸会激活转录因子 Tfeb,从而将营养应激与 TRM 编程联系起来。此外,Flcn 缺陷促进了小肠中的保护性 TRM 细胞反应。从机制上讲,Flcn-Tfeb 轴抑制了视黄酸诱导的 CCR9 迁移和转化生长因子 β (TGF-β) 介导的谱系分化编程的表达。遗传相互作用筛选显示,线粒体蛋白 Mrpl52 使早期 TRM 细胞形成成为可能,而 Acss1 在 Flcn 缺陷相关溶酶体失调下控制 TRM 细胞发育。因此,营养物质、细胞器信号传导和代谢适应之间的相互作用决定了组织免疫力。
更新日期:2024-10-14
中文翻译:

CRISPR 筛选揭示了影响肠道组织驻留记忆 CD8+ T 细胞形成的营养依赖性溶酶体和线粒体节点
营养可用性和细胞器生物学指导组织稳态和细胞命运,但这些过程如何协调组织免疫仍然不明确。在这里,使用 体内 CRISPR-Cas9 筛选,我们揭示了塑造 CD8+ 组织驻留记忆 T (TRM) 细胞发育的细胞器信号传导和代谢过程。TRM 细胞依赖于线粒体翻译和呼吸。相反,三个营养依赖性溶酶体信号节点 — Flcn、Rajurator 和 Rag GTP 酶 — 抑制肠道 TRM 细胞形成。消耗这些分子或氨基酸会激活转录因子 Tfeb,从而将营养应激与 TRM 编程联系起来。此外,Flcn 缺陷促进了小肠中的保护性 TRM 细胞反应。从机制上讲,Flcn-Tfeb 轴抑制了视黄酸诱导的 CCR9 迁移和转化生长因子 β (TGF-β) 介导的谱系分化编程的表达。遗传相互作用筛选显示,线粒体蛋白 Mrpl52 使早期 TRM 细胞形成成为可能,而 Acss1 在 Flcn 缺陷相关溶酶体失调下控制 TRM 细胞发育。因此,营养物质、细胞器信号传导和代谢适应之间的相互作用决定了组织免疫力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号