Acta Neuropathologica ( IF 9.3 ) Pub Date : 2024-10-11 , DOI: 10.1007/s00401-024-02810-1 Nicholas Sweeney, Tae Yeon Kim, Cody T. Morrison, Liangping Li, Diana Acosta, Jiawen Liang, Nithin V. Datla, Julie A. Fitzgerald, Haoran Huang, Xianglan Liu, Gregory Huang Tan, Min Wu, Kate Karelina, Chelsea E. Bray, Zachary M. Weil, Douglas W. Scharre, Geidy E. Serrano, Takashi Saito, Takaomi C. Saido, Thomas G. Beach, Olga N. Kokiko-Cochran, Jonathan P. Godbout, Gail V. W. Johnson, Hongjun Fu
|
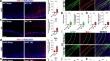
Growing evidence supports that early- or middle-life traumatic brain injury (TBI) is a risk factor for developing Alzheimer’s disease (AD) and AD-related dementia (ADRD). Nevertheless, the molecular mechanisms underlying TBI-induced AD-like pathology and cognitive deficits remain unclear. In this study, we found that a single TBI (induced by controlled cortical impact) reduced the expression of BCL2-associated athanogene 3 (BAG3) in neurons and oligodendrocytes, which is associated with decreased proteins related to the autophagy-lysosome pathway (ALP) and increased hyperphosphorylated tau (ptau) accumulation in excitatory neurons and oligodendrocytes, gliosis, synaptic dysfunction, and cognitive deficits in wild-type (WT) and human tau knock-in (hTKI) mice. These pathological changes were also found in human cases with a TBI history and exaggerated in human AD cases with TBI. The knockdown of BAG3 significantly inhibited autophagic flux, while overexpression of BAG3 significantly increased it in vitro. Specific overexpression of neuronal BAG3 in the hippocampus attenuated AD-like pathology and cognitive deficits induced by TBI in hTKI mice, which is associated with increased ALP-related proteins. Our data suggest that targeting neuronal BAG3 may be a therapeutic strategy for preventing or reducing AD-like pathology and cognitive deficits induced by TBI.
中文翻译:

神经元 BAG3 通过调节自噬-溶酶体通路减弱创伤性脑损伤诱导的 tau 过度磷酸化、突触功能障碍和认知缺陷
越来越多的证据表明,早期或中期创伤性脑损伤 (TBI) 是发展阿尔茨海默病 (AD) 和 AD 相关痴呆 (ADRD) 的危险因素。然而,TBI 诱导的 AD 样病理和认知缺陷的分子机制仍不清楚。在这项研究中,我们发现单个 TBI(由受控皮质影响诱导)降低了神经元和少突胶质细胞中 BCL2 相关肌群基因 3 (BAG3) 的表达,这与自噬-溶酶体途径 (ALP) 相关的蛋白质减少和兴奋性神经元和少突胶质细胞中的过度磷酸化 tau (ptau) 积累增加、胶质增生、突触功能障碍和野生型 (WT) 和人 tau 敲入 (hTKI) 小鼠的认知缺陷有关。这些病理变化也见于有 TBI 病史的人类病例,而在患有 TBI 的人类 AD 病例中则被夸大了。BAG3 的敲低显着抑制了自噬通量,而 BAG3 的过表达在体外显着增加了自噬通量。神经元 BAG3 在海马体中的特异性过表达减轻了 hTKI 小鼠 TBI 诱导的 AD 样病理和认知缺陷,这与 ALP 相关蛋白的增加有关。我们的数据表明,靶向神经元 BAG3 可能是预防或减少 TBI 诱导的 AD 样病理和认知缺陷的治疗策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号