Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unlocking dynamic intermetallic synergy: Ir/Ni alloy nanoparticles catalyze CO2 hydrogenation to formic acid in ionic liquid environments
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-12 , DOI: 10.1016/j.jcat.2024.115791 Rodrigo Webber, Muhammad I. Qadir, Marcus V. Castegnaro, Renato B. Pontes, Kácris I.M. da Silva, Jairton Dupont
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-12 , DOI: 10.1016/j.jcat.2024.115791 Rodrigo Webber, Muhammad I. Qadir, Marcus V. Castegnaro, Renato B. Pontes, Kácris I.M. da Silva, Jairton Dupont
|
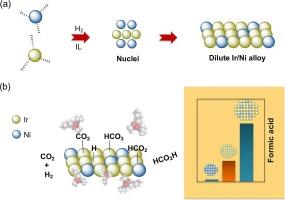
The Bimetallic Ir/Ni alloy nanoparticles (∼2.0 nm) are prepared in 1-n-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (BMIm.NTf2 ) ionic liquid, which have a unique architecture, featuring an Ir-rich alloy core surrounded by a Ni-rich alloy. The prepared bimetallic NPs exhibited efficient activity for the hydrogenation of CO2 to formic acid (1.02 M) with TOFs of 151.7 h−1 as compared to their counterparts; Ir NPs (TOF 38.5 h−1 ) and Ni NPs (TOF 0) in 1-n-butyl-3-methylimidazolium acetate (BMIm.OAc) IL solutions. This synergistic metal dilution effect arises from the inter-particle heterogeneities present in our Ir/Ni alloy NPs, causing alterations in surface structure and composition with an increase in the number of d-band holes. It offers a diverse population of active sites and tunes the adsorbate migration between reactive intermediates. The BMIm.OAc IL representes multifunctional roles, acting as a buffer and creating an IL-solvent cage around the NPs, reactants, and reactive intermediates. This alters the entropy by increasing the number of microstates, overcoming thermodynamic limitations. The synchrotron X-ray photoelectron spectroscopy and soft-XANES analyses reveal that the Ir/Ni NPs have variable electron density, the NPs surface have lower electron density than those at inner regions, which enhance the number of valence vacancies. The dilution of iridium with nickel in our Ir/Ni NPs attributes the abundance of d-band holes and O-p holes to the high oxidation state at the surface, and increases the presence of lattice vacancies. These distinct characteristics of NPs significantly boost the FA formation as compared to their counterparts. DFT calculations confirmed that the formation of FA follows the hydrogenation of HCO3 * reactive intermediates.
中文翻译:

释放动态金属间化合物协同作用:Ir/Ni 合金纳米颗粒在离子液体环境中催化 CO2 加氢生成甲酸
双金属Ir/Ni合金纳米颗粒(∼2.0 nm)是在1-n-丁基-3-甲基咪唑鎓双(三氟甲烷磺酰基)酰亚胺(BMIm.NTf2)离子液体中制备的,其特点是富Ir合金芯被富镍合金包围。制备的双金属 NPs 表现出将 CO2 加氢为甲酸 (1.02 M) 的有效活性,与同类相比,TOF 为 151.7 h-1;Ir NPs (TOF 38.5 h-1) 和 Ni NPs (TOF 0) 溶于 1-正丁基-3-甲基咪唑乙酸铵 (BMIm.OAc) IL 溶液中。这种协同金属稀释效应是由我们的 Ir/Ni 合金 NP 中存在的颗粒间异质性引起的,随着 d 波段空穴数量的增加,导致表面结构和成分发生变化。它提供了多种活性位点,并调节了反应性中间体之间的吸附物迁移。BMIm.OAc IL 代表多功能作用,充当缓冲剂并在 NPs、反应物和反应性中间体周围形成 IL 溶剂笼。这通过增加微态的数量来改变熵,克服了热力学的限制。同步辐射 X 射线光电子能谱和软 XANES 分析表明,Ir/Ni NPs 具有可变的电子密度,NPs 表面的电子密度低于内部区域的电子密度,这增加了价空位的数量。在我们的 Ir/Ni NP 中铱与镍的稀释将 d 带空穴和 O-p 空穴的丰度归因于表面的高氧化态,并增加了晶格空位的存在。与同类产品相比,NP 的这些独特特性显着促进了 FA 的形成。DFT 计算证实,FA 的形成是在 HCO3* 反应性中间体氢化之后进行的。
更新日期:2024-10-12
中文翻译:

释放动态金属间化合物协同作用:Ir/Ni 合金纳米颗粒在离子液体环境中催化 CO2 加氢生成甲酸
双金属Ir/Ni合金纳米颗粒(∼2.0 nm)是在1-n-丁基-3-甲基咪唑鎓双(三氟甲烷磺酰基)酰亚胺(BMIm.NTf2)离子液体中制备的,其特点是富Ir合金芯被富镍合金包围。制备的双金属 NPs 表现出将 CO2 加氢为甲酸 (1.02 M) 的有效活性,与同类相比,TOF 为 151.7 h-1;Ir NPs (TOF 38.5 h-1) 和 Ni NPs (TOF 0) 溶于 1-正丁基-3-甲基咪唑乙酸铵 (BMIm.OAc) IL 溶液中。这种协同金属稀释效应是由我们的 Ir/Ni 合金 NP 中存在的颗粒间异质性引起的,随着 d 波段空穴数量的增加,导致表面结构和成分发生变化。它提供了多种活性位点,并调节了反应性中间体之间的吸附物迁移。BMIm.OAc IL 代表多功能作用,充当缓冲剂并在 NPs、反应物和反应性中间体周围形成 IL 溶剂笼。这通过增加微态的数量来改变熵,克服了热力学的限制。同步辐射 X 射线光电子能谱和软 XANES 分析表明,Ir/Ni NPs 具有可变的电子密度,NPs 表面的电子密度低于内部区域的电子密度,这增加了价空位的数量。在我们的 Ir/Ni NP 中铱与镍的稀释将 d 带空穴和 O-p 空穴的丰度归因于表面的高氧化态,并增加了晶格空位的存在。与同类产品相比,NP 的这些独特特性显着促进了 FA 的形成。DFT 计算证实,FA 的形成是在 HCO3* 反应性中间体氢化之后进行的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号