当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and stereoselectivity in metal and enzyme catalyzed carbene insertion into X–H and C(sp2)–H bonds
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-10-11 , DOI: 10.1039/d4cs00742e Reena Balhara, Ritwika Chatterjee, Garima Jindal
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-10-11 , DOI: 10.1039/d4cs00742e Reena Balhara, Ritwika Chatterjee, Garima Jindal
|
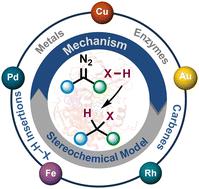
Constructing highly proficient C–X (X = O, N, S, etc.) and C–C bonds by leveraging TMs (transition metals) (Fe, Cu, Pd, Rh, Au, etc.) and enzymes to catalyze carbene insertion into X–H/C(sp2)–H is a highly versatile strategy. This is primarily achieved through the in situ generation of metal carbenes from the interaction of TMs with diazo compounds. Over the last few decades, significant advancements have been made, encompassing a wide array of X–H bond insertions using various TMs. These reactions typically favor a stepwise ionic pathway where the nucleophilic attack on the metal carbene leads to the generation of a metal ylide species. This intermediate marks a critical juncture in the reaction cascade, presenting multiple avenues for proton transfer to yield the X–H inserted product. The mechanism of C(sp2)–H insertion reactions closely resembles those of X–H insertion reactions and thus have been included here. A major development in carbene insertion reactions has been the use of engineered enzymes as catalysts. Since the seminal report of a non-natural “carbene transferase” by Arnold in 2013, “P411”, several heme-based enzymes have been reported in the literature to catalyze various abiological carbene insertion reactions into C(sp2)–H, N–H and S–H bonds. These enzymes possess an extraordinary ability to regulate the orientation and conformations of reactive intermediates, facilitating stereoselective carbene transfers. However, the absence of a suitable stereochemical model has impeded the development of asymmetric reactions employing a lone chiral catalyst, including enzymes. There is a pressing need to investigate alternative mechanisms and models to enhance our comprehension of stereoselectivity in these processes, which will be crucial for advancing the fields of asymmetric synthesis and biocatalysis. The current review aims to provide details on the mechanistic aspects of the asymmetric X–H and C(sp2)–H insertion reactions catalyzed by Fe, Cu, Pd, Rh, Au, and enzymes, focusing on the detailed mechanism and stereochemical model. The review is divided into sections focusing on a specific X–H/C(sp2)–H bond type catalyzed by different TMs and enzymes.
中文翻译:

金属和酶催化的卡宾插入 X-H 和 C(sp2)-H 键的机制和立体选择性
通过利用 TM(过渡金属)(Fe、Cu、Pd、Rh、Au 等)和酶催化卡宾插入 X-H/C(sp2)-H 来构建高度熟练的 C-X(X = O、N、S 等)和 C-C 键是一种高度通用的策略。这主要是通过 TM 与重氮化合物相互作用原位生成金属卡宾来实现的。在过去的几十年里,已经取得了重大进展,包括使用各种 TM 进行广泛的 X-H 键插入。这些反应通常有利于逐步离子途径,其中对金属卡宾的亲核攻击导致金属酰化物物质的产生。该中间体标志着反应级联反应的关键时刻,为质子转移提供了多种途径,以产生 X-H 插入产物。C(sp2)–H 插入反应的机制与 X–H 插入反应的机制非常相似,因此已包括在此处。卡宾插入反应的一个主要发展是使用工程酶作为催化剂。自 Arnold 于 2013 年开创性地报道了一种非天然“卡宾转移酶”“P411”以来,文献中已经报道了几种基于血红素的酶,它们可以催化各种非生物卡宾插入反应到 C(sp2)-H、N-H 和 S-H 键中。这些酶具有调节反应性中间体的方向和构象的非凡能力,可促进立体选择性卡宾转移。然而,缺乏合适的立体化学模型阻碍了使用孤手性催化剂(包括酶)的不对称反应的发展。 迫切需要研究替代机制和模型,以增强我们对这些过程中立体选择性的理解,这对于推进不对称合成和生物催化领域至关重要。目前的综述旨在提供有关由 Fe、Cu、Pd、Rh、Au 和酶催化的不对称 X-H 和 C(sp2)-H 插入反应的机理方面的详细信息,重点介绍详细的机理和立体化学模型。本综述分为几个部分,重点介绍由不同 TM 和酶催化的特定 X-H/C(sp2)-H 键类型。
更新日期:2024-10-11
中文翻译:

金属和酶催化的卡宾插入 X-H 和 C(sp2)-H 键的机制和立体选择性
通过利用 TM(过渡金属)(Fe、Cu、Pd、Rh、Au 等)和酶催化卡宾插入 X-H/C(sp2)-H 来构建高度熟练的 C-X(X = O、N、S 等)和 C-C 键是一种高度通用的策略。这主要是通过 TM 与重氮化合物相互作用原位生成金属卡宾来实现的。在过去的几十年里,已经取得了重大进展,包括使用各种 TM 进行广泛的 X-H 键插入。这些反应通常有利于逐步离子途径,其中对金属卡宾的亲核攻击导致金属酰化物物质的产生。该中间体标志着反应级联反应的关键时刻,为质子转移提供了多种途径,以产生 X-H 插入产物。C(sp2)–H 插入反应的机制与 X–H 插入反应的机制非常相似,因此已包括在此处。卡宾插入反应的一个主要发展是使用工程酶作为催化剂。自 Arnold 于 2013 年开创性地报道了一种非天然“卡宾转移酶”“P411”以来,文献中已经报道了几种基于血红素的酶,它们可以催化各种非生物卡宾插入反应到 C(sp2)-H、N-H 和 S-H 键中。这些酶具有调节反应性中间体的方向和构象的非凡能力,可促进立体选择性卡宾转移。然而,缺乏合适的立体化学模型阻碍了使用孤手性催化剂(包括酶)的不对称反应的发展。 迫切需要研究替代机制和模型,以增强我们对这些过程中立体选择性的理解,这对于推进不对称合成和生物催化领域至关重要。目前的综述旨在提供有关由 Fe、Cu、Pd、Rh、Au 和酶催化的不对称 X-H 和 C(sp2)-H 插入反应的机理方面的详细信息,重点介绍详细的机理和立体化学模型。本综述分为几个部分,重点介绍由不同 TM 和酶催化的特定 X-H/C(sp2)-H 键类型。






























 京公网安备 11010802027423号
京公网安备 11010802027423号