当前位置:
X-MOL 学术
›
J. Magnes. Alloys
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mg anode interface engineering in KNO3 electrolyte with sodium 5-sulfosalicylate as an additive for enhanced performance of Mg-air batteries
Journal of Magnesium and Alloys ( IF 15.8 ) Pub Date : 2024-10-10 , DOI: 10.1016/j.jma.2024.09.007 Guanhua Lin, Yaqing Zhou, Sandrine Zanna, Antoine Seyeux, Philippe Marcus, Jolanta Światowska
Journal of Magnesium and Alloys ( IF 15.8 ) Pub Date : 2024-10-10 , DOI: 10.1016/j.jma.2024.09.007 Guanhua Lin, Yaqing Zhou, Sandrine Zanna, Antoine Seyeux, Philippe Marcus, Jolanta Światowska
|
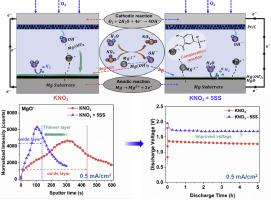
The Mg-air batteries face limitations with pronounced hydrogen evolution and low anodic utilization efficiency from Mg anodes in conventional NaCl electrolytes. The corrosion performance, surface composition, and discharge properties of commercial purity Mg anodes were thoroughly investigated in KNO3 electrolytes with and without sodium 5-sulfosalicylate and compared to NaCl electrolyte. The addition of sodium 5-sulfosalicylate to KNO3 -based electrolyte results in efficient inhibition of H2 evolution, consequently enhancing anodic utilization efficiency to 84% and specific capacity to 1844 mAh/g, compared to NaCl (24% and 534 mAh/g, respectively) under discharge condition of 10 mA/cm2 in half cell. Furthermore, the chelating ability of sodium 5-sulfosalicylate can significantly improve the Mg surface dissolution kinetics and discharge product deposition rate at the Mg anode / electrolyte interface, yielding formation of a thinner discharge layer as confirmed by time-of-flight secondary ion mass spectrometry. The discharge voltage is increased to 1.60 V, compared to 1.35 V in KNO3 at 0.5 mA/cm2 in full cell. However, higher concentration of sodium 5-sulfosalicylate can accelerate Mg anode dissolution, impeding the improvement of anodic utilization efficiency, specific capacity, and energy density. Hence, determining optimal additive concentration and current density is crucial for enhancing the discharge properties of Mg-air batteries and mitigating excessive Mg dissolution in chloride-free electrolytes.
中文翻译:

以 5-磺基水杨酸钠为添加剂的 KNO3 电解质中的 Mg 负极界面工程,用于增强 Mg-air 电池的性能
Mg-air 电池面临传统 NaCl 电解质中 Mg 负极明显的析氢和低阳极利用效率的限制。在含和不含 5-磺基水杨酸钠的 KNO3 电解液中,以及与 NaCl 电解质的比较,对商业纯度 Mg 阳极的腐蚀性能、表面组成和放电性能进行了深入研究。在 10 mA/cm2 的放电条件下,在 KNO3 基电解质中添加 5-磺基水杨酸钠可有效抑制 H2 的析出,从而将阳极利用效率提高到 84%,比容量提高到 1844 mAh/g,与 NaCl (分别为 24% 和 534 mAh/g) 相比。此外,5-磺基水杨酸钠的螯合能力可以显着改善 Mg 表面溶解动力学和放电产物在 Mg 阳极/电解质界面的沉积速率,从而形成更薄的放电层,飞行时间二次离子质谱证实。放电电压增加到 1.60 V,而 KNO3 的放电电压为 1.35 V,全电池为 0.5 mA/cm2。然而,较高浓度的 5-磺基水杨酸钠会加速 Mg 阳极的溶解,阻碍阳极利用效率、比容量和能量密度的提高。因此,确定最佳的添加剂浓度和电流密度对于增强 Mg-air 电池的放电性能和减少 Mg 在无氯电解质中的过度溶解至关重要。
更新日期:2024-10-10
中文翻译:

以 5-磺基水杨酸钠为添加剂的 KNO3 电解质中的 Mg 负极界面工程,用于增强 Mg-air 电池的性能
Mg-air 电池面临传统 NaCl 电解质中 Mg 负极明显的析氢和低阳极利用效率的限制。在含和不含 5-磺基水杨酸钠的 KNO3 电解液中,以及与 NaCl 电解质的比较,对商业纯度 Mg 阳极的腐蚀性能、表面组成和放电性能进行了深入研究。在 10 mA/cm2 的放电条件下,在 KNO3 基电解质中添加 5-磺基水杨酸钠可有效抑制 H2 的析出,从而将阳极利用效率提高到 84%,比容量提高到 1844 mAh/g,与 NaCl (分别为 24% 和 534 mAh/g) 相比。此外,5-磺基水杨酸钠的螯合能力可以显着改善 Mg 表面溶解动力学和放电产物在 Mg 阳极/电解质界面的沉积速率,从而形成更薄的放电层,飞行时间二次离子质谱证实。放电电压增加到 1.60 V,而 KNO3 的放电电压为 1.35 V,全电池为 0.5 mA/cm2。然而,较高浓度的 5-磺基水杨酸钠会加速 Mg 阳极的溶解,阻碍阳极利用效率、比容量和能量密度的提高。因此,确定最佳的添加剂浓度和电流密度对于增强 Mg-air 电池的放电性能和减少 Mg 在无氯电解质中的过度溶解至关重要。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号