当前位置:
X-MOL 学术
›
ACS Appl. Polym. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Reduction-Sensitive Brush-Arm Star Polymers via Combined Ring-Opening Metathesis Polymerization–Atom Transfer Radical Polymerization and Controlled-Release Properties
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2024-10-10 , DOI: 10.1021/acsapm.4c02427 Xuhui Liang, Yuping Liu, Zhen Dong, Nanting Qiu, Fuwei Kong, Kun Cao, Zhong-Ren Chen
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2024-10-10 , DOI: 10.1021/acsapm.4c02427 Xuhui Liang, Yuping Liu, Zhen Dong, Nanting Qiu, Fuwei Kong, Kun Cao, Zhong-Ren Chen
|
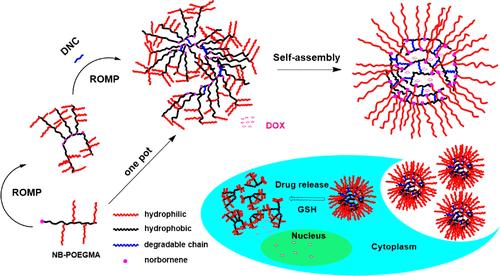
Mild reaction conditions and excellent functional group tolerance make ring-opening metathesis polymerization (ROMP) a widely used technique to synthesize functional materials. In this work, we used ROMP and atom transfer radical polymerization (ATRP) to prepare a reduction-sensitive brush-arm star polymer (BASP) drug carrier by a one-pot method and further conducted in vitro studies. First, a bifunctional norbornene-based ATRP initiator NB-Br and a reduction-sensitive dinorbornene cross-linker (DNC) were synthesized. Subsequently, NB-Br was subjected to ATRP using the hydrophilic monomer oligoethylene glycol monomethyl ether methacrylate (OEGMA) at different polymerization times to obtain the norbornenyl macromonomer NB-P(OEGMA)n with different degrees of polymerization. In the same reaction flask, NB-P(OEGMA)n underwent an ROMP reaction to generate a brush-shaped polymer. Next, DNC was added to the reaction flask, and the reaction was continued to obtain BASP. This one-pot method could successfully be used to achieve the transformation of the macromonomer to the brush-shaped polymer and subsequently to the star polymer. In vitro biological experiments demonstrated that BASP micelles exhibited reduction-triggered cleavage of disulfide bonds and degradation of polymers after coculture with 10 mM glutathione, promoting the release of intracellular doxorubicin and achieving the controlled release of drugs. We expect the findings of our study to serve as an efficient synthetic strategy to prepare carriers for biomedical applications.
中文翻译:

通过组合开环复分解聚合-原子转移自由基聚合和控释特性合成还原敏感的刷臂星形聚合物
温和的反应条件和出色的官能团耐受性使开环复分解聚合 (ROMP) 成为合成功能材料的广泛使用的技术。在这项工作中,我们使用 ROMP 和原子转移自由基聚合 (ATRP) 通过一锅法制备还原敏感刷臂星形聚合物 (BASP) 药物载体,并进一步进行了体外研究。首先,合成了基于双功能降冰片烯的 ATRP 引发剂 NB-Br 和还原敏感的降冰烯交联剂 (DNC)。随后,使用亲水性单体寡乙二醇单甲醚甲基丙烯酸酯 (OEGMA) 在不同聚合时间对 NB-Br 进行 ATRP,得到不同聚合度的降冰片烯基大分子单体 NB-P(OEGMA)n。在同一反应瓶中,NB-P(OEGMA)n 进行 ROMP 反应,生成刷状聚合物。接下来,将 DNC 添加到反应瓶中,并继续反应以获得 BASP。这种一锅法可以成功地用于实现大分子单体向刷状聚合物的转变,然后转化为星形聚合物。体外生物实验表明,BASP 胶束与 10 mM 谷胱甘肽共培养后表现出还原触发的二硫键裂解和聚合物降解,促进细胞内阿霉素的释放并实现药物的控释。我们希望我们的研究结果能够作为一种有效的合成策略,为生物医学应用准备载体。
更新日期:2024-10-10
中文翻译:

通过组合开环复分解聚合-原子转移自由基聚合和控释特性合成还原敏感的刷臂星形聚合物
温和的反应条件和出色的官能团耐受性使开环复分解聚合 (ROMP) 成为合成功能材料的广泛使用的技术。在这项工作中,我们使用 ROMP 和原子转移自由基聚合 (ATRP) 通过一锅法制备还原敏感刷臂星形聚合物 (BASP) 药物载体,并进一步进行了体外研究。首先,合成了基于双功能降冰片烯的 ATRP 引发剂 NB-Br 和还原敏感的降冰烯交联剂 (DNC)。随后,使用亲水性单体寡乙二醇单甲醚甲基丙烯酸酯 (OEGMA) 在不同聚合时间对 NB-Br 进行 ATRP,得到不同聚合度的降冰片烯基大分子单体 NB-P(OEGMA)n。在同一反应瓶中,NB-P(OEGMA)n 进行 ROMP 反应,生成刷状聚合物。接下来,将 DNC 添加到反应瓶中,并继续反应以获得 BASP。这种一锅法可以成功地用于实现大分子单体向刷状聚合物的转变,然后转化为星形聚合物。体外生物实验表明,BASP 胶束与 10 mM 谷胱甘肽共培养后表现出还原触发的二硫键裂解和聚合物降解,促进细胞内阿霉素的释放并实现药物的控释。我们希望我们的研究结果能够作为一种有效的合成策略,为生物医学应用准备载体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号