当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Disulfide/Trisulfide Core-Cross-Linked Polycarbonate Nanocarriers for Intracellular Reduction-Triggered Drug Release
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-10-09 , DOI: 10.1021/acsmacrolett.4c00443 Jiye Zhao, Dongdong Wang, Xi Zhang, Yaodong Di, Shuai Yang, Lesan Yan
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-10-09 , DOI: 10.1021/acsmacrolett.4c00443 Jiye Zhao, Dongdong Wang, Xi Zhang, Yaodong Di, Shuai Yang, Lesan Yan
|
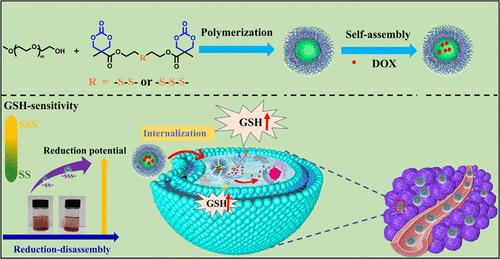
Polymeric nanocarriers have attracted significant attention in the field of anticancer drug delivery due to their unique advantages. However, designing nanocarriers that can maintain stability in the bloodstream while achieving specific drug release within tumor cells remains a major challenge. To address this issue, constructing reversible cross-linked polymeric nanocarriers that are sensitive to the intracellular reducible glutathione (GSH) characteristic of the tumor microenvironment is a promising strategy. Based on this, we designed and synthesized two novel six-membered bicyclic carbonate monomers containing disulfide (DSBC) and trisulfide (TSBC) bonds. Through a one-step ring-opening polymerization, a series of reduction-sensitive polycarbonate copolymers (i.e., PEG–PDSBC and PEG–PTSBC) were prepared, and doxorubicin (DOX)-loaded nanoparticles were fabricated using a nanoprecipitation method. The in vitro drug release behaviors of these nanoparticles were systematically investigated. The results showed that these polymers, due to the cross-linked structure formed by the ring-opening polymerization of their bicyclic monomers, could self-assemble into stable nanoparticles. Under different concentrations of glutathione, DOX-loaded PEG–PTSBC nanoparticles demonstrated faster drug release, indicating more optimized intracellular drug release properties. Further cytotoxicity experiments revealed that both types of blank nanoparticles exhibited good biocompatibility with the 4T1 and NIH-3T3 cells. Fluorescence microscopy and flow cytometry results further indicated that DOX-loaded PEG–PTSBC nanoparticles released more drugs in 4T1 cells, significantly inhibiting tumor cell growth compared with DOX-loaded PEG–PDSBC nanoparticles, with no noticeable difference in NIH-3T3 normal cells. In conclusion, this study suggests that trisulfide cross-linked polycarbonate-based nanocarriers hold promise as an anticancer drug delivery system that combines stability in the bloodstream with specific intracellular drug release, offering new insights for the development of novel, efficient, and safe anticancer nanomedicines.
中文翻译:

二硫键/三硫键交联聚碳酸酯纳米载体的制备,用于细胞内还原触发的药物释放
聚合物纳米载体因其独特的优势而在抗癌药物递送领域引起了极大的关注。然而,设计能够保持血流稳定性同时在肿瘤细胞内实现特异性药物释放的纳米载体仍然是一个重大挑战。为了解决这个问题,构建对肿瘤微环境的细胞内可还原谷胱甘肽 (GSH) 特征敏感的可逆交联聚合物纳米载体是一种很有前途的策略。基于此,我们设计合成了两种含有二硫键 (DSBC) 和三硫键 (TSBC) 的新型六元双元碳酸盐单体。通过一步开环聚合,制备了一系列还原敏感的聚碳酸酯共聚物 (即 PEG-PDSBC 和 PEG-PTSBC),并使用纳米沉淀法制备了负载多柔比星 (DOX) 的纳米颗粒。系统研究了这些纳米颗粒的体外药物释放行为。结果表明,这些聚合物由于其双环单体的开环聚合形成交联结构,可以自组装成稳定的纳米颗粒。在不同浓度的谷胱甘肽下,负载 DOX 的 PEG-PTSBC 纳米颗粒表现出更快的药物释放,表明细胞内药物释放特性更优化。进一步的细胞毒性实验表明,两种类型的空白纳米颗粒与 4T1 和 NIH-3T3 细胞均表现出良好的生物相容性。 荧光显微镜和流式细胞术结果进一步表明,与 DOX 负载的 PEG-PDSBC 纳米颗粒相比,负载 DOX 的 PEG-PTSBC 纳米颗粒在 4T1 细胞中释放了更多的药物,显着抑制了肿瘤细胞的生长,在 NIH-3T3 正常细胞中没有明显差异。总之,这项研究表明,基于三硫键交联聚碳酸酯的纳米载体有望作为一种抗癌药物递送系统,该系统将血液中的稳定性与特定的细胞内药物释放相结合,为开发新型、高效和安全的抗癌纳米药物提供了新的见解。
更新日期:2024-10-09
中文翻译:

二硫键/三硫键交联聚碳酸酯纳米载体的制备,用于细胞内还原触发的药物释放
聚合物纳米载体因其独特的优势而在抗癌药物递送领域引起了极大的关注。然而,设计能够保持血流稳定性同时在肿瘤细胞内实现特异性药物释放的纳米载体仍然是一个重大挑战。为了解决这个问题,构建对肿瘤微环境的细胞内可还原谷胱甘肽 (GSH) 特征敏感的可逆交联聚合物纳米载体是一种很有前途的策略。基于此,我们设计合成了两种含有二硫键 (DSBC) 和三硫键 (TSBC) 的新型六元双元碳酸盐单体。通过一步开环聚合,制备了一系列还原敏感的聚碳酸酯共聚物 (即 PEG-PDSBC 和 PEG-PTSBC),并使用纳米沉淀法制备了负载多柔比星 (DOX) 的纳米颗粒。系统研究了这些纳米颗粒的体外药物释放行为。结果表明,这些聚合物由于其双环单体的开环聚合形成交联结构,可以自组装成稳定的纳米颗粒。在不同浓度的谷胱甘肽下,负载 DOX 的 PEG-PTSBC 纳米颗粒表现出更快的药物释放,表明细胞内药物释放特性更优化。进一步的细胞毒性实验表明,两种类型的空白纳米颗粒与 4T1 和 NIH-3T3 细胞均表现出良好的生物相容性。 荧光显微镜和流式细胞术结果进一步表明,与 DOX 负载的 PEG-PDSBC 纳米颗粒相比,负载 DOX 的 PEG-PTSBC 纳米颗粒在 4T1 细胞中释放了更多的药物,显着抑制了肿瘤细胞的生长,在 NIH-3T3 正常细胞中没有明显差异。总之,这项研究表明,基于三硫键交联聚碳酸酯的纳米载体有望作为一种抗癌药物递送系统,该系统将血液中的稳定性与特定的细胞内药物释放相结合,为开发新型、高效和安全的抗癌纳米药物提供了新的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号