当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Type I Photosensitizer-Polymersome Boosts Reactive Oxygen Species Generation by Forcing H-Aggregation for Amplifying STING Immunotherapy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-10-09 , DOI: 10.1021/jacs.4c09831 Zhixiong Wang, Wen Ma, Zhen Yang, Dale O. Kiesewetter, Yicong Wu, Lixin Lang, Guofeng Zhang, Sofia Nakuchima, Jiji Chen, Yijun Su, Sue Han, Ling-Gang Wu, Albert J. Jin, Wei Huang
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-10-09 , DOI: 10.1021/jacs.4c09831 Zhixiong Wang, Wen Ma, Zhen Yang, Dale O. Kiesewetter, Yicong Wu, Lixin Lang, Guofeng Zhang, Sofia Nakuchima, Jiji Chen, Yijun Su, Sue Han, Ling-Gang Wu, Albert J. Jin, Wei Huang
|
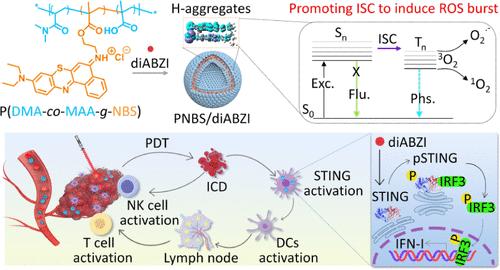
Activation of the innate immune Stimulator of Interferon Genes (STING) pathway potentiates antitumor immunity. However, delivering STING agonists systemically to tumors presents a formidable challenge, and resistance to STING monotherapy has emerged in clinical trials with diminishing natural killer (NK) cell proliferation. Here, we encapsulated the STING agonist diABZI within polymersomes containing a Type I photosensitizer (NBS), creating a nanoagonist (PNBS/diABZI) for highly responsive tumor immunotherapy. This structure promoted H-aggregation and intersystem crossing of NBS, resulting in a ∼ 3-fold amplification in superoxide anion and singlet oxygen generation. The photodynamic therapy directly damaged hypoxia tumor cells and stimulated the proliferation of NK cells and cytotoxic T lymphocytes, thereby sensitizing STING immunotherapy. A single systemic intravenous administration of PNBS/diABZI eradicated orthotopic mammary tumors in murine models, achieving long-term antitumor immune memory to inhibit tumor recurrence and metastasis and significantly improving long-term tumor-free survival. This work provides a design rule for boosting reactive oxygen species production by promoting the intersystem crossing process, highlighting the potential of Type I photosensitizer-polymer vehicles for augmenting STING immunotherapy.
中文翻译:

I 型光敏剂聚合物通过强制 H 聚集来增强 STING 免疫疗法,从而促进活性氧的产生
先天免疫干扰素基因刺激剂 (STING) 通路的激活可增强抗肿瘤免疫力。然而,将 STING 激动剂全身递送到肿瘤是一项艰巨的挑战,并且在自然杀伤 (NK) 细胞增殖减少的临床试验中已经出现了对 STING 单一疗法的耐药性。在这里,我们将 STING 激动剂 diABZI 封装在含有 I 型光敏剂 (NBS) 的聚合物体中,从而产生用于高反应性肿瘤免疫治疗的纳米激动剂 (PNBS/diABZI)。这种结构促进了 NBS 的 H 聚集和系统间交叉,导致超氧阴离子和单线态氧的产生扩增 ∼ 3 倍。光动力疗法直接损伤缺氧肿瘤细胞,刺激 NK 细胞和细胞毒性 T 淋巴细胞增殖,从而使 STING 免疫疗法敏感。PNBS/diABZI 的单次全身静脉内给药根除小鼠模型中的原位乳腺肿瘤,实现长期抗肿瘤免疫记忆,抑制肿瘤复发和转移,显著提高长期无肿瘤生存率。这项工作提供了一种通过促进系统间交叉过程来促进活性氧产生的设计规则,突出了 I 型光敏剂-聚合物载体增强 STING 免疫治疗的潜力。
更新日期:2024-10-09
中文翻译:

I 型光敏剂聚合物通过强制 H 聚集来增强 STING 免疫疗法,从而促进活性氧的产生
先天免疫干扰素基因刺激剂 (STING) 通路的激活可增强抗肿瘤免疫力。然而,将 STING 激动剂全身递送到肿瘤是一项艰巨的挑战,并且在自然杀伤 (NK) 细胞增殖减少的临床试验中已经出现了对 STING 单一疗法的耐药性。在这里,我们将 STING 激动剂 diABZI 封装在含有 I 型光敏剂 (NBS) 的聚合物体中,从而产生用于高反应性肿瘤免疫治疗的纳米激动剂 (PNBS/diABZI)。这种结构促进了 NBS 的 H 聚集和系统间交叉,导致超氧阴离子和单线态氧的产生扩增 ∼ 3 倍。光动力疗法直接损伤缺氧肿瘤细胞,刺激 NK 细胞和细胞毒性 T 淋巴细胞增殖,从而使 STING 免疫疗法敏感。PNBS/diABZI 的单次全身静脉内给药根除小鼠模型中的原位乳腺肿瘤,实现长期抗肿瘤免疫记忆,抑制肿瘤复发和转移,显著提高长期无肿瘤生存率。这项工作提供了一种通过促进系统间交叉过程来促进活性氧产生的设计规则,突出了 I 型光敏剂-聚合物载体增强 STING 免疫治疗的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号