Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural mechanism of HP1⍺-dependent transcriptional repression and chromatin compaction
Structure ( IF 4.4 ) Pub Date : 2024-10-08 , DOI: 10.1016/j.str.2024.09.013 Vladyslava Sokolova, Jacob Miratsky, Vladimir Svetlov, Michael Brenowitz, John Vant, Tyler S. Lewis, Kelly Dryden, Gahyun Lee, Shayan Sarkar, Evgeny Nudler, Abhishek Singharoy, Dongyan Tan
Structure ( IF 4.4 ) Pub Date : 2024-10-08 , DOI: 10.1016/j.str.2024.09.013 Vladyslava Sokolova, Jacob Miratsky, Vladimir Svetlov, Michael Brenowitz, John Vant, Tyler S. Lewis, Kelly Dryden, Gahyun Lee, Shayan Sarkar, Evgeny Nudler, Abhishek Singharoy, Dongyan Tan
|
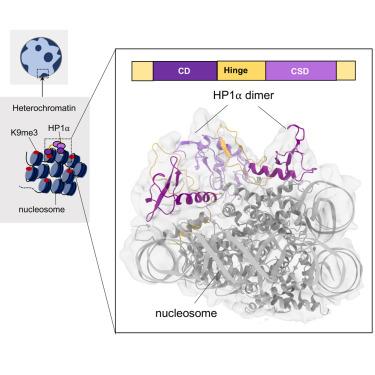
Heterochromatin protein 1 (HP1) plays a central role in establishing and maintaining constitutive heterochromatin. However, the mechanisms underlying HP1-nucleosome interactions and their contributions to heterochromatin functions remain elusive. Here, we present the cryoelectron microscopy (cryo-EM) structure of an HP1α dimer bound to an H2A.Z-nucleosome, revealing two distinct HP1α-nucleosome interfaces. The primary HP1α binding site is located at the N terminus of histone H3, specifically at the trimethylated lysine 9 (K9me3) region, while a secondary binding site is situated near histone H2B, close to nucleosome superhelical location 4 (SHL4). Our biochemical data further demonstrates that HP1α binding influences the dynamics of DNA on the nucleosome. It promotes DNA unwrapping near the nucleosome entry and exit sites while concurrently restricting DNA accessibility in the vicinity of SHL4. Our study offers a model for HP1α-mediated heterochromatin maintenance and gene silencing. It also sheds light on the H3K9me-independent role of HP1 in responding to DNA damage.
中文翻译:

HP1⍺ 依赖性转录抑制和染色质压缩的结构机制
异染色质蛋白 1 (HP1) 在建立和维持组成型异染色质中起着核心作用。然而,HP1-核小体相互作用的潜在机制及其对异染色质功能的贡献仍然难以捉摸。在这里,我们展示了与 H 2 A 结合的 HP1α 二聚体的冷冻电子显微镜 (cryo-EM) 结构。Z 核小体,揭示了两个不同的 HP1α-核小体界面。主要 HP1α 结合位点位于组蛋白 H3 的 N 末端,特别是在三甲基化赖氨酸 9 (K9me3) 区域,而次级结合位点位于组蛋白 H2B 附近,靠近核小体超螺旋位置 4 (SHL4)。我们的生化数据进一步表明,HP1α 结合会影响核小体上 DNA 的动力学。它促进核小体进入和出口位点附近的 DNA 解包,同时限制 SHL4 附近的 DNA 可及性。我们的研究为 HP1α 介导的异染色质维持和基因沉默提供了一个模型。它还阐明了 HP1 在响应 DNA 损伤中不依赖 H3K9me 的作用。
更新日期:2024-10-08
中文翻译:

HP1⍺ 依赖性转录抑制和染色质压缩的结构机制
异染色质蛋白 1 (HP1) 在建立和维持组成型异染色质中起着核心作用。然而,HP1-核小体相互作用的潜在机制及其对异染色质功能的贡献仍然难以捉摸。在这里,我们展示了与 H 2 A 结合的 HP1α 二聚体的冷冻电子显微镜 (cryo-EM) 结构。Z 核小体,揭示了两个不同的 HP1α-核小体界面。主要 HP1α 结合位点位于组蛋白 H3 的 N 末端,特别是在三甲基化赖氨酸 9 (K9me3) 区域,而次级结合位点位于组蛋白 H2B 附近,靠近核小体超螺旋位置 4 (SHL4)。我们的生化数据进一步表明,HP1α 结合会影响核小体上 DNA 的动力学。它促进核小体进入和出口位点附近的 DNA 解包,同时限制 SHL4 附近的 DNA 可及性。我们的研究为 HP1α 介导的异染色质维持和基因沉默提供了一个模型。它还阐明了 HP1 在响应 DNA 损伤中不依赖 H3K9me 的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号