当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
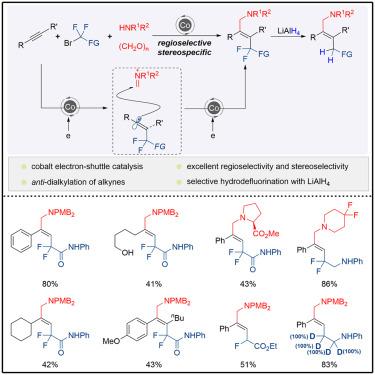
Electron-shuttle catalysis enables regioselective and stereospecific dialkylation of alkynes
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-08 , DOI: 10.1016/j.checat.2024.101129 Zhiting Wang, Changqing Rao, Hanmin Huang
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-08 , DOI: 10.1016/j.checat.2024.101129 Zhiting Wang, Changqing Rao, Hanmin Huang
|
The dicarbofunctionalization of alkynes with unsaturated electrophiles and alkyl halides proceeding via the oxidative cyclometallation pathway offers a promising tool to synthesize multi-substituted alkenes. However, the inherent formation of cyclometallic intermediates is limited to the formation of cis products. We demonstrate here that the anti-dialkylation of alkynes with alkyl halides and unsaturated iminium is accessible via sequential radical addition promoted by cobalt electron-shuttle catalysis. This process exhibits broad substrate scope and high functional group compatibility, providing a straightforward and efficient approach to densely functionalized trans-alkenes. The newly developed facile hydrodefluorination enabled by simple LiAlH4 further enhances the practicability of this procedure. Mechanistic studies revealed that bypassing the formation of alkenyl metal species is beneficial to obtain excellent stereoselectivity.
中文翻译:

电子穿梭催化能够实现炔烃的区域选择性和立体特异性二烷基化
通过氧化环金属化途径进行的炔烃与不饱和亲电子试剂和卤代烷的二碳官能化提供了合成多取代烯烃的有前景的工具。然而,环金属中间体的固有形成仅限于顺式产物的形成。我们在这里证明,通过钴电子穿梭催化促进的顺序自由基加成,可以用烷基卤和不饱和亚胺进行炔烃的反二烷基化。该过程表现出广泛的底物范围和高官能团兼容性,为密集官能化反式烯烃提供了一种简单有效的方法。新开发的通过简单的LiAlH 4实现的简易加氢脱氟进一步增强了该过程的实用性。机理研究表明,绕过烯基金属物种的形成有利于获得优异的立体选择性。
更新日期:2024-10-08
中文翻译:

电子穿梭催化能够实现炔烃的区域选择性和立体特异性二烷基化
通过氧化环金属化途径进行的炔烃与不饱和亲电子试剂和卤代烷的二碳官能化提供了合成多取代烯烃的有前景的工具。然而,环金属中间体的固有形成仅限于顺式产物的形成。我们在这里证明,通过钴电子穿梭催化促进的顺序自由基加成,可以用烷基卤和不饱和亚胺进行炔烃的反二烷基化。该过程表现出广泛的底物范围和高官能团兼容性,为密集官能化反式烯烃提供了一种简单有效的方法。新开发的通过简单的LiAlH 4实现的简易加氢脱氟进一步增强了该过程的实用性。机理研究表明,绕过烯基金属物种的形成有利于获得优异的立体选择性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号