当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blood‐Brain Barrier‐Penetrating Metal‐Organic Framework Antioxidant Nanozymes for Targeted Ischemic Stroke Therapy
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2024-10-07 , DOI: 10.1002/adhm.202402376
Qing Chen 1 , Jin Wang 2, 3 , Xiaoxing Xiong 2, 4 , Junyang Chen 1 , Bo Wang 1 , Haixia Yang 5 , Jianliang Zhou 1 , Hongping Deng 6 , Lijuan Gu 2, 3 , Jian Tian 1, 5
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2024-10-07 , DOI: 10.1002/adhm.202402376
Qing Chen 1 , Jin Wang 2, 3 , Xiaoxing Xiong 2, 4 , Junyang Chen 1 , Bo Wang 1 , Haixia Yang 5 , Jianliang Zhou 1 , Hongping Deng 6 , Lijuan Gu 2, 3 , Jian Tian 1, 5
Affiliation
|
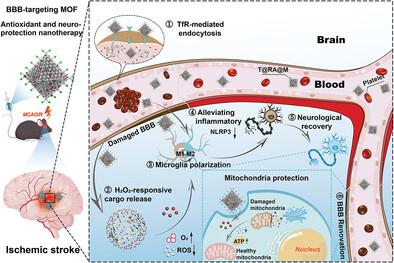
Overproduction of reactive oxygen species (ROS) during reperfusion in ischemic stroke (IS) severely impedes neuronal survival and results in high rates of morbidity and disability. The effective blood‐brain barrier (BBB) penetration and brain delivery of antioxidative agents remain the biggest challenge in treating ischemic reperfusion‐induced cerebrovascular and neural injury. In this study, a metal‐organic framework (MOF) nanozyme (MIL‐101‐NH2 (Fe/Cu)) with ROS scavenging activities to encapsulate neuroprotective agent rapamycin is fabricated and decorating the exterior with BBB‐targeting protein ligands (transferrin), thereby realizing enhanced drug retention and controlled release within ischemic lesions for the synergistic treatment of IS. Through the receptor‐mediated transcellular pathway, the transferrin‐coated MOF nanoparticles achieved efficient transport across the BBB and targeted accumulation at the cerebral ischemic injury site of mice with middle cerebral artery occlusion/reperfusion (MCAO/R), wherein the nanocarrier exhibited catalytic activities of ROS decomposition into O2 and H2 O2 ‐responsive rapamycin release. By its BBB‐targeting, antioxidative, anti‐inflammatory, and antiapoptotic properties, the MOF nanosystem addressed multiple pathological factors of IS and realized remarkable neuroprotective effects, leading to the substantial reduction of cerebral infarction volume and accelerated recovery of nerve functions in the MCAO/R mouse model. This MOF‐based nanomedicine provides valuable design principles for effective IS therapy with multi‐mechanism synergies.
中文翻译:

用于靶向缺血性脑卒中治疗的血脑屏障穿透金属有机框架抗氧化纳米酶
缺血性卒中 (IS) 再灌注过程中活性氧 (ROS) 的过量产生会严重阻碍神经元存活,并导致高发病率和残疾率。抗氧化剂的有效血脑屏障 (BBB) 渗透和脑递送仍然是治疗缺血再灌注诱导的脑血管和神经损伤的最大挑战。在本研究中,制备了一种具有 ROS 清除活性的金属有机框架 (MOF) 纳米酶 (MIL-101-NH2(Fe/Cu)) 以包埋神经保护剂雷帕霉素,并用 BBB 靶向蛋白配体(转铁蛋白)装饰外部,从而实现缺血病灶内药物保留和控释,用于协同治疗 IS。通过受体介导的跨细胞途径,转铁蛋白包被的 MOF 纳米颗粒实现了跨 BBB 的有效运输,并在大脑中动脉闭塞/再灌注 (MCAO/R) 小鼠的脑缺血损伤部位靶向积累,其中纳米载体表现出 ROS 分解成 O2 和 H2O2 反应性雷帕霉素释放的催化活性。通过其 BBB 靶向、抗氧化、抗炎和抗凋亡特性,MOF 纳米系统解决了 IS 的多种病理因素并实现了显着的神经保护作用,导致脑梗死体积的大幅减少并加速了 MCAO/R 小鼠模型中的神经功能恢复。这种基于 MOF 的纳米药物为具有多机制协同作用的有效 IS 治疗提供了有价值的设计原则。
更新日期:2024-10-07
中文翻译:

用于靶向缺血性脑卒中治疗的血脑屏障穿透金属有机框架抗氧化纳米酶
缺血性卒中 (IS) 再灌注过程中活性氧 (ROS) 的过量产生会严重阻碍神经元存活,并导致高发病率和残疾率。抗氧化剂的有效血脑屏障 (BBB) 渗透和脑递送仍然是治疗缺血再灌注诱导的脑血管和神经损伤的最大挑战。在本研究中,制备了一种具有 ROS 清除活性的金属有机框架 (MOF) 纳米酶 (MIL-101-NH2(Fe/Cu)) 以包埋神经保护剂雷帕霉素,并用 BBB 靶向蛋白配体(转铁蛋白)装饰外部,从而实现缺血病灶内药物保留和控释,用于协同治疗 IS。通过受体介导的跨细胞途径,转铁蛋白包被的 MOF 纳米颗粒实现了跨 BBB 的有效运输,并在大脑中动脉闭塞/再灌注 (MCAO/R) 小鼠的脑缺血损伤部位靶向积累,其中纳米载体表现出 ROS 分解成 O2 和 H2O2 反应性雷帕霉素释放的催化活性。通过其 BBB 靶向、抗氧化、抗炎和抗凋亡特性,MOF 纳米系统解决了 IS 的多种病理因素并实现了显着的神经保护作用,导致脑梗死体积的大幅减少并加速了 MCAO/R 小鼠模型中的神经功能恢复。这种基于 MOF 的纳米药物为具有多机制协同作用的有效 IS 治疗提供了有价值的设计原则。

































 京公网安备 11010802027423号
京公网安备 11010802027423号