当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elevated muramyl dipeptide by sialic acid-facilitated postantibiotic pathobiont expansion contributes to gut dysbiosis-induced mastitis in mice
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-05 , DOI: 10.1016/j.jare.2024.09.023 Min Qiu, Cong Ye, Lijuan Bao, Keyi Wu, Yihong Zhao, Xiaotong Zhao, Ruibo Tang, Ruping Shang, Shan Shang, Chongshan Yuan, Xiaoyu Hu, Naisheng Zhang, Yunhe Fu, Jun Wang, Caijun Zhao
中文翻译:

唾液酸促进的抗生素后致病性病变扩增导致胞壁酰二肽升高,导致小鼠肠道菌群失调诱导的乳腺炎
在对抗生素暴露的反应中,肠道菌群失调不仅是病原体感染的危险因素,也是促进致病菌扩张的危险因素,导致肠道和远处器官的炎症反应增加。然而,如何监管这一过程尚未完全阐明。
在这项研究中,我们调查了唾液酸(一种宿主来源的碳水化合物)在远处器官肠道菌群失调衍生炎症发病机制中的作用。
氨苄青霉素 (Amp) 诱导的肠道营养不良小鼠用 N-羟基神经氨酸 (Neu5Gc) 和 N-乙酰神经氨酸 (Neu5Ac) 处理 3 周,以评估唾液酸在乳腺炎中的作用。使用 16S rRNA 测序、转录组学和采用多种分子方法探索唾液酸调节乳腺炎的潜在机制。
Neu5Ac 和 Neu5Gc 的给药加剧了肠道菌群失调诱导的乳腺炎和全身炎症。唾液酸也加剧了 Amp 引起的肠道菌群失调。值得注意的是,在 Neu5Ac-和 Neu5Gc 处理的肠道营养不良小鼠中观察到肠球菌扩张增加,这与炎症标志物呈正相关。用盲肠球菌 (E. cecorum) 治疗小鼠加重了肠道菌群失调诱导的乳腺炎。在机械上,唾液酸促进的 E. cecorum 扩增促进了胞壁酰二肽 (MDP) 的释放,从而通过激活 NOD2-RIP2-NF-κB 轴诱导炎症反应。
总的来说,我们的数据揭示了唾液酸促进的抗生素后致病体扩张在肠道菌群失调相关炎症中的作用,突出了通过调节 MDP-NOD2-RIP2 轴来预防疾病的潜在策略。
更新日期:2024-10-06
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-05 , DOI: 10.1016/j.jare.2024.09.023 Min Qiu, Cong Ye, Lijuan Bao, Keyi Wu, Yihong Zhao, Xiaotong Zhao, Ruibo Tang, Ruping Shang, Shan Shang, Chongshan Yuan, Xiaoyu Hu, Naisheng Zhang, Yunhe Fu, Jun Wang, Caijun Zhao
|
Introduction
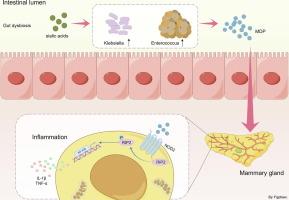
In responses to antibiotics exposure, gut dysbiosis is a risk factor not only for pathogen infection but also for facilitating pathobiont expansion, resulting in increased inflammatory responses in the gut and distant organs. However, how this process is regulated has not been fully elucidated.Objectives
In this study, we investigated the role of sialic acid, a host-derived carbohydrate, in the pathogenesis of gut dysbiosis-derived inflammation in distant organs.Methods
Ampicillin (Amp)-induced gut dysbiotic mice were treated with N-glycolylneuraminic acid (Neu5Gc) and N-acetylneuraminic acid (Neu5Ac) for three weeks to assess the role of sialic acids in mastitis. The underlying mechanism by which sialic acids regulate mastitis was explored using 16S rRNA sequencing, transcriptomics and employed multiple molecular approaches.Results
Administration of Neu5Ac and Neu5Gc exacerbated gut dysbiosis-induced mastitis and systemic inflammation. The gut dysbiosis caused by Amp was also aggravated by sialic acid. Notably, increased Enterococcus expansion, which was positively correlated with inflammatory markers, was observed in both Neu5Ac- and Neu5Gc-treated gut dysbiotic mice. Treatment of mice with Enterococcus cecorum (E. cecorum) aggravated gut dysbiosis-induced mastitis. Mechanically, sialic acid-facilitated E. cecorum expansion promoted muramyl dipeptide (MDP) release, which induced inflammatory responses by activating the NOD2-RIP2-NF-κB axis.Conclusions
Collectively, our data reveal a role of sialic acid-facilitated postantibiotic pathobiont expansion in gut dysbiosis-associated inflammation, highlighting a potential strategy for disease prevention by regulating the MDP-NOD2-RIP2 axis.中文翻译:

唾液酸促进的抗生素后致病性病变扩增导致胞壁酰二肽升高,导致小鼠肠道菌群失调诱导的乳腺炎
介绍
在对抗生素暴露的反应中,肠道菌群失调不仅是病原体感染的危险因素,也是促进致病菌扩张的危险因素,导致肠道和远处器官的炎症反应增加。然而,如何监管这一过程尚未完全阐明。
目标
在这项研究中,我们调查了唾液酸(一种宿主来源的碳水化合物)在远处器官肠道菌群失调衍生炎症发病机制中的作用。
方法
氨苄青霉素 (Amp) 诱导的肠道营养不良小鼠用 N-羟基神经氨酸 (Neu5Gc) 和 N-乙酰神经氨酸 (Neu5Ac) 处理 3 周,以评估唾液酸在乳腺炎中的作用。使用 16S rRNA 测序、转录组学和采用多种分子方法探索唾液酸调节乳腺炎的潜在机制。
结果
Neu5Ac 和 Neu5Gc 的给药加剧了肠道菌群失调诱导的乳腺炎和全身炎症。唾液酸也加剧了 Amp 引起的肠道菌群失调。值得注意的是,在 Neu5Ac-和 Neu5Gc 处理的肠道营养不良小鼠中观察到肠球菌扩张增加,这与炎症标志物呈正相关。用盲肠球菌 (E. cecorum) 治疗小鼠加重了肠道菌群失调诱导的乳腺炎。在机械上,唾液酸促进的 E. cecorum 扩增促进了胞壁酰二肽 (MDP) 的释放,从而通过激活 NOD2-RIP2-NF-κB 轴诱导炎症反应。
结论
总的来说,我们的数据揭示了唾液酸促进的抗生素后致病体扩张在肠道菌群失调相关炎症中的作用,突出了通过调节 MDP-NOD2-RIP2 轴来预防疾病的潜在策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号