当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pinpointing Conditions for a Metabolic Origin of Life: Underlying Mechanisms and the Role of Coenzymes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-10-05 , DOI: 10.1021/acs.accounts.4c00423 Joris Zimmermann, Emilie Werner, Shunjiro Sodei, Joseph Moran
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-10-05 , DOI: 10.1021/acs.accounts.4c00423 Joris Zimmermann, Emilie Werner, Shunjiro Sodei, Joseph Moran
|
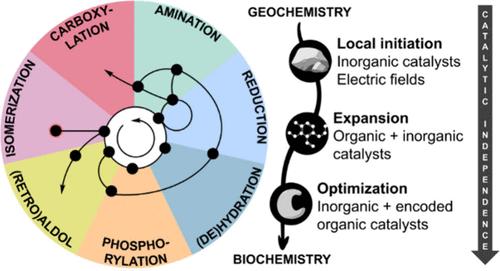
Famously found written on the blackboard of physicist Richard Feynman after his death was the phrase, “What I cannot create, I do not understand.” From this perspective, recreating the origin of life in the lab is a necessary condition for achieving a deep theoretical understanding of biology. The “metabolism-first” hypothesis is one of the leading frameworks for the origin of life. A complex self-organized reaction network is thought to have been driven into existence as a chemical path of least resistance to release free energy in the environment that could otherwise not be dissipated, rerouting energy from planetary processes to organic chemistry. To increase in complexity, the reaction network, initially under catalysis provided by its geochemical environment, must have produced organic catalysts that pruned the existing flux through the network or expanded it in new directions. This boot-strapping process would gradually lessen the dependence on the initial catalytic environment and allow the reaction network to persist using catalysts of its own making. Eventually, this process leads to the seemingly inseparable interdependence at the heart of biology between catalysts (coenzymes, enzymes, genes) and the metabolic pathways that synthesize them. Experimentally, the primary challenge is to recreate the conditions where such a network emerged. However, the near infinite number of microenvironments and sources of energy available on the early Earth or elsewhere poses an enormous combinatorial challenge. To constrain the search, our lab has been surveying conditions where the reactions making up the core of some of the most ancient chemolithoautotrophic metabolisms, which consist of only a small number of repeating chemical mechanisms, occur nonenzymatically. To give a fresh viewpoint in the first part of this account, we have organized the results of our search (along with important results from other laboratories) by reaction mechanism, rather than by pathway. We expect that identifying a common set of conditions for each type of reaction mechanism will help pinpoint the conditions for the emergence of a self-organized reaction network resembling core metabolism. Many of the reaction mechanisms were found to occur in a wide variety of nonenzymatic conditions. Others, such as carboxylate phosphorylation and C–C bond formation from CO2, were found to be the most constraining, and thus help narrow the scope of environments where a reaction network could emerge. In the second part of this account, we highlight examples where small molecules produced by metabolism, known as coenzymes, mediate nonenzymatic chemistry of the type needed for the coenzyme’s own synthesis or that turn on new reactivity of interest for expanding a hypothetical protometabolic network. These examples often feature cooperativity between small organic coenzymes and metal ions, recapitulating the transition from inorganic to organic catalysis during the origin of life. Overall, the most interesting conditions are those containing a reducing potential equivalent to H2 gas (electrochemical or H2 itself), Fe in both reduced and more oxidized forms (possibly with other metals like Ni) and localized strong electric fields. Environments that satisfy these criteria simultaneously will be of prime interest for reconstructing a metabolic origin of life.
中文翻译:

确定生命代谢起源的条件:潜在机制和辅酶的作用
物理学家理查德·费曼 (Richard Feynman) 去世后,在他的黑板上发现了一句著名的话:“我无法创造的,我不理解。从这个角度来看,在实验室中重现生命的起源是实现对生物学有深入理论理解的必要条件。“新陈代谢优先”假说是生命起源的主要框架之一。一个复杂的自组织反应网络被认为是作为一种化学路径而存在的,它对释放环境中本来无法消散的自由能的抵抗力最小,将能量从行星过程转移到有机化学。为了增加复杂性,最初在其地球化学环境提供的催化下,反应网络必须产生有机催化剂,这些催化剂通过网络修剪现有的通量或将其扩展到新的方向。这种引导过程将逐渐减少对初始催化环境的依赖,并允许反应网络使用自己制造的催化剂持续存在。最终,这个过程导致催化剂(辅酶、酶、基因)和合成它们的代谢途径之间似乎不可分割的相互依存关系,这是生物学的核心。从实验上讲,主要挑战是重建这种网络出现的条件。然而,早期地球或其他地方可用的微环境和能源数量几乎无限,这构成了巨大的组合挑战。为了限制搜索,我们的实验室一直在调查构成一些最古老的化学自养代谢核心的反应以非酶方式发生的条件,这些代谢仅由少量重复的化学机制组成。 为了在本文的第一部分提供新的视角,我们按反应机制而不是途径组织了我们的检索结果(以及其他实验室的重要结果)。我们预计,为每种类型的反应机制确定一组共同的条件将有助于确定出现类似于核心代谢的自组织反应网络的条件。发现许多反应机制发生在各种非酶促条件下。其他因素,例如羧酸盐磷酸化和 CO2 的 C-C 键形成,被发现是最受限制的,因此有助于缩小可能出现反应网络的环境范围。在本文的第二部分,我们重点介绍了新陈代谢产生的小分子(称为辅酶)介导辅酶自身合成所需的非酶化学类型的例子,或者激发新的感兴趣反应性以扩展假设的原生代谢网络。这些例子通常以小有机辅酶和金属离子之间的协同性为特征,概括了生命起源过程中从无机催化到有机催化的转变。总的来说,最有趣的条件是那些含有相当于 H2 气体(电化学或 H2 本身)、还原和更氧化形式的 Fe(可能与 Ni 等其他金属一起)和局部强电场的条件。同时满足这些标准的环境将是重建生命代谢起源的主要兴趣。
更新日期:2024-10-05
中文翻译:

确定生命代谢起源的条件:潜在机制和辅酶的作用
物理学家理查德·费曼 (Richard Feynman) 去世后,在他的黑板上发现了一句著名的话:“我无法创造的,我不理解。从这个角度来看,在实验室中重现生命的起源是实现对生物学有深入理论理解的必要条件。“新陈代谢优先”假说是生命起源的主要框架之一。一个复杂的自组织反应网络被认为是作为一种化学路径而存在的,它对释放环境中本来无法消散的自由能的抵抗力最小,将能量从行星过程转移到有机化学。为了增加复杂性,最初在其地球化学环境提供的催化下,反应网络必须产生有机催化剂,这些催化剂通过网络修剪现有的通量或将其扩展到新的方向。这种引导过程将逐渐减少对初始催化环境的依赖,并允许反应网络使用自己制造的催化剂持续存在。最终,这个过程导致催化剂(辅酶、酶、基因)和合成它们的代谢途径之间似乎不可分割的相互依存关系,这是生物学的核心。从实验上讲,主要挑战是重建这种网络出现的条件。然而,早期地球或其他地方可用的微环境和能源数量几乎无限,这构成了巨大的组合挑战。为了限制搜索,我们的实验室一直在调查构成一些最古老的化学自养代谢核心的反应以非酶方式发生的条件,这些代谢仅由少量重复的化学机制组成。 为了在本文的第一部分提供新的视角,我们按反应机制而不是途径组织了我们的检索结果(以及其他实验室的重要结果)。我们预计,为每种类型的反应机制确定一组共同的条件将有助于确定出现类似于核心代谢的自组织反应网络的条件。发现许多反应机制发生在各种非酶促条件下。其他因素,例如羧酸盐磷酸化和 CO2 的 C-C 键形成,被发现是最受限制的,因此有助于缩小可能出现反应网络的环境范围。在本文的第二部分,我们重点介绍了新陈代谢产生的小分子(称为辅酶)介导辅酶自身合成所需的非酶化学类型的例子,或者激发新的感兴趣反应性以扩展假设的原生代谢网络。这些例子通常以小有机辅酶和金属离子之间的协同性为特征,概括了生命起源过程中从无机催化到有机催化的转变。总的来说,最有趣的条件是那些含有相当于 H2 气体(电化学或 H2 本身)、还原和更氧化形式的 Fe(可能与 Ni 等其他金属一起)和局部强电场的条件。同时满足这些标准的环境将是重建生命代谢起源的主要兴趣。































 京公网安备 11010802027423号
京公网安备 11010802027423号