当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Serum metabolite biomarkers for the early diagnosis and monitoring of age-related macular degeneration
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-05 , DOI: 10.1016/j.jare.2024.10.001 Shengjie Li, Yichao Qiu, Yingzhu Li, Jianing Wu, Ning Yin, Jun Ren, Mingxi Shao, Jian Yu, Yunxiao Song, Xinghuai Sun, Shunxiang Gao, Wenjun Cao
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-05 , DOI: 10.1016/j.jare.2024.10.001 Shengjie Li, Yichao Qiu, Yingzhu Li, Jianing Wu, Ning Yin, Jun Ren, Mingxi Shao, Jian Yu, Yunxiao Song, Xinghuai Sun, Shunxiang Gao, Wenjun Cao
|
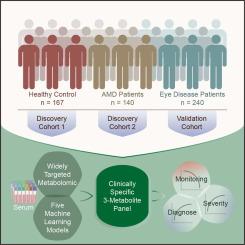
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness worldwide, with significant challenges for early diagnosis and treatment.
中文翻译:

用于年龄相关性黄斑变性早期诊断和监测的血清代谢物生物标志物
年龄相关性黄斑变性 (AMD) 是全球不可逆失明的主要原因,对早期诊断和治疗提出了重大挑战。
更新日期:2024-10-05
中文翻译:

用于年龄相关性黄斑变性早期诊断和监测的血清代谢物生物标志物
年龄相关性黄斑变性 (AMD) 是全球不可逆失明的主要原因,对早期诊断和治疗提出了重大挑战。

































 京公网安备 11010802027423号
京公网安备 11010802027423号