当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterometallic Molecular and Ionic Isomers
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-10-03 , DOI: 10.1021/acs.inorgchem.4c03849 Yuxuan Zhang, Zheng Wei, Haixiang Han, Joyce Chang, Samantha Stegman, Tieyan Chang, Yu-Sheng Chen, John F. Berry, Evgeny V. Dikarev
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-10-03 , DOI: 10.1021/acs.inorgchem.4c03849 Yuxuan Zhang, Zheng Wei, Haixiang Han, Joyce Chang, Samantha Stegman, Tieyan Chang, Yu-Sheng Chen, John F. Berry, Evgeny V. Dikarev
|
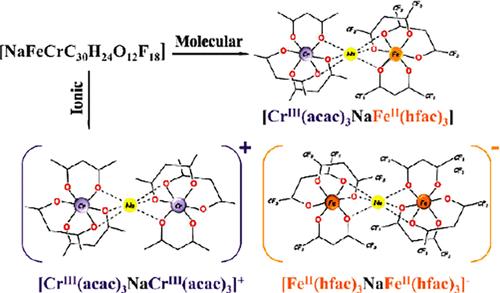
Numerous descriptions of structural isomerism in metal complexes do not list any molecular vs ionic isomers. At the same time, one of the most striking examples of structural isomerism in organic chemistry is molecular urea, which has the same atomic composition as the chemically distinct ionic ammonium cyanate. This iconic organic couple now meets its inorganic heterometallic counterpart. We introduce a new class of structural isomers, molecular vs ionic, that can be consummated in complex and coordinatively unsaturated polynuclear/heterometallic compounds. We report inorganic molecular and ionic isomers of the composition [NaCrFe (acac)3(hfac)3] (acac = acetylacetonate; hfac = hexafluoroacetylacetonate). Heterometallic molecular [CrIII(acac)3-Na-FeII(hfac)3] (1m) and ionic {[CrIII(acac)3-Na-CrIII(acac)3]+[FeII(hfac)3-Na-FeII(hfac)3]−} (1i) isomers have been isolated in pure form and characterized. While both ions are heterobimetallic trinuclear entities, the neutral counterpart is a heterotrimetallic trinuclear molecule. The two isomers exhibit distinctly different characteristics in terms of solubility, volatility, mass spectrometry ionization, and thermal behavior. Unambiguous assignment of the positions and oxidation/spin states of the Periodic Table neighbors, Fe and Cr, in both isomers have been made by a combination of characterization techniques that include synchrotron X-ray resonant diffraction, synchrotron X-ray fluorescence spectroscopy, Mössbauer spectroscopy, and DART mass spectrometry. The transformation between the two isomers that does take place in solutions of noncoordinating solvents has also been tested.
中文翻译:

异金属分子和离子异构体
对金属配合物中结构异构的许多描述都没有列出任何分子异构体与离子异构体。同时,有机化学中结构异构最引人注目的例子之一是分子尿素,它与化学上不同的离子氰酸铵具有相同的原子组成。这对标志性的有机对现在遇到了它的无机异质金属对应物。我们介绍了一类新的结构异构体,分子与离子,它可以在复杂且配位不饱和的多核/异金属化合物中完成。我们报道了组合物 [NaCrFe (acac)3(hfac)3] (acac = 乙酰丙糖酸盐;hfac = 六氟乙酰丙酮酸盐)的无机分子和离子异构体。杂金属分子 [CrIII(acac)3-Na-FeII(hfac)3] (1m) 和离子 {[CrIII(acac)3-Na-Cr III(acac)3]+[FeII(hfac)3-Na-Fe II(hfac)3]−} (1i) 异构体已以纯形式分离并表征。虽然两种离子都是异质双金属三核实体,但中性对应物是异质三金属三核分子。这两种异构体在溶解度、挥发性、质谱电离和热行为方面表现出明显不同的特性。元素周期表相邻的 Fe 和 Cr 在两种异构体中的位置和氧化/自旋态的分配是通过结合表征技术(包括同步加速器 X 射线共振衍射、同步加速器 X 射线荧光光谱、穆斯堡尔光谱和 DART 质谱)来实现的。 在非配位溶剂溶液中发生的两种异构体之间的转变也已经过测试。
更新日期:2024-10-03
中文翻译:

异金属分子和离子异构体
对金属配合物中结构异构的许多描述都没有列出任何分子异构体与离子异构体。同时,有机化学中结构异构最引人注目的例子之一是分子尿素,它与化学上不同的离子氰酸铵具有相同的原子组成。这对标志性的有机对现在遇到了它的无机异质金属对应物。我们介绍了一类新的结构异构体,分子与离子,它可以在复杂且配位不饱和的多核/异金属化合物中完成。我们报道了组合物 [NaCrFe (acac)3(hfac)3] (acac = 乙酰丙糖酸盐;hfac = 六氟乙酰丙酮酸盐)的无机分子和离子异构体。杂金属分子 [CrIII(acac)3-Na-FeII(hfac)3] (1m) 和离子 {[CrIII(acac)3-Na-Cr III(acac)3]+[FeII(hfac)3-Na-Fe II(hfac)3]−} (1i) 异构体已以纯形式分离并表征。虽然两种离子都是异质双金属三核实体,但中性对应物是异质三金属三核分子。这两种异构体在溶解度、挥发性、质谱电离和热行为方面表现出明显不同的特性。元素周期表相邻的 Fe 和 Cr 在两种异构体中的位置和氧化/自旋态的分配是通过结合表征技术(包括同步加速器 X 射线共振衍射、同步加速器 X 射线荧光光谱、穆斯堡尔光谱和 DART 质谱)来实现的。 在非配位溶剂溶液中发生的两种异构体之间的转变也已经过测试。






























 京公网安备 11010802027423号
京公网安备 11010802027423号