当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salidroside sensitizes Triple-negative breast cancer to ferroptosis by SCD1-mediated lipogenesis and NCOA4-mediated ferritinophagy
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-09-29 , DOI: 10.1016/j.jare.2024.09.027 Guiqin Huang, Yawen Cai, Menghui Ren, Xiaoyu Zhang, Yu Fu, Run Cheng, Yingdi Wang, Mingxing Miao, Lingpeng Zhu, Tianhua Yan
中文翻译:

红景天苷通过 SCD1 介导的脂肪生成和 NCOA4 介导的铁蛋白自噬使三阴性乳腺癌对铁死亡敏感
三阴性乳腺癌(TNBC)是乳腺癌导致女性死亡的主要原因。文献已证实红景天苷 (Sal) 在治疗 TNBC 方面的益处。然而,关于 Sal 锚定的 TNBC 潜在治疗靶点和机制的研究仍然有限。
本研究旨在探讨Sal对抗TNBC的主要靶点和潜在机制。
整合网络药理学、生物信息学和机器学习算法策略来研究 Sal 行为在 TNBC 中的作用、潜在靶点和机制。选择MDA-MB-231细胞和荷瘤裸鼠进行体外和体内实验。使用 CCK-8、LDH 测试和 Calcein-AM/PI 染色测定细胞活力和细胞毒性。使用谷胱甘肽、谷胱甘肽过氧化物酶、丙二醛 (MDA)、C11-BODIPY 581/591 探针和 FerroOrange 染料探索抗氧化防御、脂质过氧化和铁代谢。谷胱甘肽过氧化物酶 4 (GPX4) 或硬脂酰辅酶 A 去饱和酶 1 (SCD1) 过表达或核受体共激活剂 4 (NCOA4) 缺陷来证明 Sal 对 TNBC 的作用机制。
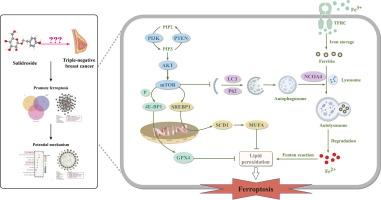
预测结果证实,在Sal和TNBC中鉴定出22个与铁死亡相关的基因,揭示了Sal作用于TNBC的潜在机制与铁死亡有关。此外,通过功能富集分析,这些基因主要涉及mTOR、PI3K/AKT和自噬信号通路。体外验证结果证实,Sal 通过提高细胞内 Fe 2+和脂质过氧化来调节铁死亡,从而抑制 TNBC 细胞增殖。从机制上讲,Sal 通过抑制 PI3K/AKT/mTOR 轴使 TNBC 细胞对铁死亡敏感,从而抑制 SCD1 介导的单不饱和脂肪酸脂肪生成以诱导脂质过氧化,另外促进 NCOA4 介导的铁蛋白自噬以增加细胞内 Fe 2+含量。 GPX4 或 SCD1 过度表达或 NCOA4 缺陷结果进一步支持了我们的机制研究。体内实验证实,Sal 对于通过诱导铁死亡来减缓肿瘤生长至关重要。
总体而言,本研究阐明了与铁死亡密切相关的 TNBC 发病机制,并确定了 TNBC 的潜在生物标志物。同时,该研究阐明,Sal 通过 SCD1 介导的脂肪生成和 NCOA4 介导的铁蛋白自噬(受 PI3K/AKT/mTOR 信号通路的调节)使 TNBC 对铁死亡敏感。我们的研究结果为应用Sal治疗TNBC提供了理论依据。
更新日期:2024-10-03
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-09-29 , DOI: 10.1016/j.jare.2024.09.027 Guiqin Huang, Yawen Cai, Menghui Ren, Xiaoyu Zhang, Yu Fu, Run Cheng, Yingdi Wang, Mingxing Miao, Lingpeng Zhu, Tianhua Yan
|
Introduction
Triple-negative breast cancer (TNBC) is the primary cause of breast cancer-induced death in women. Literature has confirmed the benefits of Salidroside (Sal) in treating TNBC. However, the study about potential therapeutic targets and mechanisms of Sal-anchored TNBC remains limited.Objective
This study was designed to explore the main targets and potential mechanisms of Sal against TNBC.Methods
Network pharmacology, bioinformatics, and machine learning algorithm strategies were integrated to examine the role, potential targets, and mechanisms of the Sal act in TNBC. MDA-MB-231 cells and tumor-bearing nude mice were chosen for in vitro and in vivo experimentation. Cell viability and cytotoxicity were determined using CCK-8, LDH test, and Calcein-AM/PI staining. Antioxidant defense, lipid peroxidation, and iron metabolism were explored using glutathione, glutathione peroxidase, malondialdehyde (MDA), C11-BODIPY 581/591 probe, and FerroOrange dye. Glutathione peroxidase 4 (GPX4) or stearoyl-CoA desaturase 1 (SCD1) overexpression or nuclear receptor co-activator 4 (NCOA4) deficiency was performed to demonstrate the mechanism of Sal on TNBC.Results
The prediction results confirmed that 22 ferroptosis-related genes were identified in Sal and TNBC, revealing that the potential mechanism of the Sal act on TNBC was linked with ferroptosis. Besides, these genes were mainly involved in the mTOR, PI3K/AKT, and autophagy signaling pathway by functional enrichment analysis. The in vitro validation results confirmed that Sal inhibited TNBC cell proliferation by modulating ferroptosis via elevation of intracellular Fe2+ and lipid peroxidation. Mechanistically, Sal sensitized TNBC cells to ferroptosis by inhibiting the PI3K/AKT/mTOR axis, thereby suppressing SCD1-mediated lipogenesis of monounsaturated fatty acids to induce lipid peroxidation, additionally facilitating NCOA4-mediated ferritinophagy to increase intracellular Fe2+ content. The GPX4 or SCD1 overexpression or NCOA4 deficiency results further supported our mechanistic studies. In vivo experimentation confirmed that Sal is vital for slowing down tumor growth by inducing ferroptosis.Conclusions
Overall, this study elucidates TNBC pathogenesis closely linked to ferroptosis and identifies potential biomarkers in TNBC. Meanwhile, the study elucidates that Sal sensitizes TNBC to ferroptosis by SCD1-mediated lipogenesis and NCOA4-mediated ferritinophagy, regulated by PI3K/AKT/mTOR signaling pathways. Our findings provide a theoretical basis for applying Sal to treat TNBC.中文翻译:

红景天苷通过 SCD1 介导的脂肪生成和 NCOA4 介导的铁蛋白自噬使三阴性乳腺癌对铁死亡敏感
介绍
三阴性乳腺癌(TNBC)是乳腺癌导致女性死亡的主要原因。文献已证实红景天苷 (Sal) 在治疗 TNBC 方面的益处。然而,关于 Sal 锚定的 TNBC 潜在治疗靶点和机制的研究仍然有限。
客观的
本研究旨在探讨Sal对抗TNBC的主要靶点和潜在机制。
方法
整合网络药理学、生物信息学和机器学习算法策略来研究 Sal 行为在 TNBC 中的作用、潜在靶点和机制。选择MDA-MB-231细胞和荷瘤裸鼠进行体外和体内实验。使用 CCK-8、LDH 测试和 Calcein-AM/PI 染色测定细胞活力和细胞毒性。使用谷胱甘肽、谷胱甘肽过氧化物酶、丙二醛 (MDA)、C11-BODIPY 581/591 探针和 FerroOrange 染料探索抗氧化防御、脂质过氧化和铁代谢。谷胱甘肽过氧化物酶 4 (GPX4) 或硬脂酰辅酶 A 去饱和酶 1 (SCD1) 过表达或核受体共激活剂 4 (NCOA4) 缺陷来证明 Sal 对 TNBC 的作用机制。
结果
预测结果证实,在Sal和TNBC中鉴定出22个与铁死亡相关的基因,揭示了Sal作用于TNBC的潜在机制与铁死亡有关。此外,通过功能富集分析,这些基因主要涉及mTOR、PI3K/AKT和自噬信号通路。体外验证结果证实,Sal 通过提高细胞内 Fe 2+和脂质过氧化来调节铁死亡,从而抑制 TNBC 细胞增殖。从机制上讲,Sal 通过抑制 PI3K/AKT/mTOR 轴使 TNBC 细胞对铁死亡敏感,从而抑制 SCD1 介导的单不饱和脂肪酸脂肪生成以诱导脂质过氧化,另外促进 NCOA4 介导的铁蛋白自噬以增加细胞内 Fe 2+含量。 GPX4 或 SCD1 过度表达或 NCOA4 缺陷结果进一步支持了我们的机制研究。体内实验证实,Sal 对于通过诱导铁死亡来减缓肿瘤生长至关重要。
结论
总体而言,本研究阐明了与铁死亡密切相关的 TNBC 发病机制,并确定了 TNBC 的潜在生物标志物。同时,该研究阐明,Sal 通过 SCD1 介导的脂肪生成和 NCOA4 介导的铁蛋白自噬(受 PI3K/AKT/mTOR 信号通路的调节)使 TNBC 对铁死亡敏感。我们的研究结果为应用Sal治疗TNBC提供了理论依据。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号