当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkylaluminum Complexes Featuring Bridged Bis-Formylfluorenimide Ligands for Hydroboration of Aldehyde, Ketone, and Imines
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-10-03 , DOI: 10.1021/acs.inorgchem.4c03158 Chaoqun Wang, Mengna Huang, Hui Miao, Chenxu Liu, Zhibiao Qin, Wenning Ma, Mengmeng Han, Junjie Yu, Yongmin Li, Biao Wei, Zheng Chen
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-10-03 , DOI: 10.1021/acs.inorgchem.4c03158 Chaoqun Wang, Mengna Huang, Hui Miao, Chenxu Liu, Zhibiao Qin, Wenning Ma, Mengmeng Han, Junjie Yu, Yongmin Li, Biao Wei, Zheng Chen
|
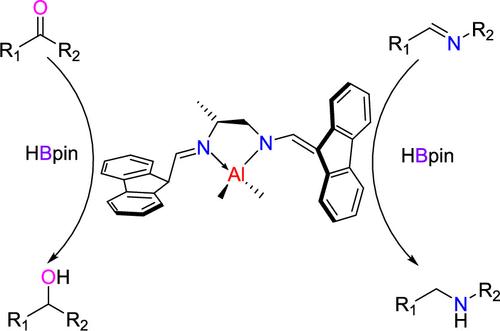
Three bis-formylfluorenimide ligands with different bridging groups were designed and synthesized, leading to the successful preparation of six novel alkylaluminum complexes through their reaction with alkylaluminum reagents (AlMe3 or AlEt3). Complexes 1 and 2 were obtained by the reaction of 1,2-propylene-bridged diamine (L1) with AlMe3 or AlEt3. By reacting 1,2-cyclohexylene-bridged diamine (L2) with AlMe3 or AlEt3 to obtain complexes 3 and 4. The above ligands formed a bidentate four-coordinate structure with alkylaluminum, which involved the elimination of one alkyl group as the ligand reacted with alkylaluminum. The complexes 5 and 6 were synthesized through the reaction of 1,2-phenylene-bridged diamine (L3) with AlEt3 in toluene or tetrahydrofuran. Notably, L3 exhibited unique reactivity compared with the other ligands, which formed a tridentate four-coordinated structure when reacting with alkylaluminum. The formation of the tridentate complex resulted from the introduction of a benzimidazole derivative or tetrahydrofuran (THF) molecule along with the elimination of two alkyl groups during its coordination with alkylaluminum. All complexes were characterized via 1H NMR, 13C NMR, and elemental analysis, with structural determination confirmed through X-ray. Furthermore, the catalytic activity in the hydroboration reaction of aldehyde, ketone, and imines was investigated with these complexes as catalysts. Among them, complex 1 demonstrated excellent catalytic performance (up to 99% yield) and broad substrate compatibility (more than 30 substrates) at low catalyst loading (1 mol %) under mild reaction conditions.
中文翻译:

烷基铝配合物,具有桥式双甲酰基芴酰亚胺配体,用于醛、酮和亚胺的氢硼化反应
设计合成了三种具有不同桥接基团的双甲酰基芴酰亚胺配体,通过与烷基铝试剂 (AlMe3 或 AlEt3) 反应成功制备了 6 种新型烷基铝配合物。通过 1,2-丙烯桥二胺 (L1) 与 AlMe3 或 AlEt3 反应获得复合物 1 和 2。通过将 1,2-环己基桥二胺 (L2) 与 AlMe3 或 AlEt3 反应得到络合物 3 和 4。上述配体与烷基铝形成双齿四配位结构,当配体与烷基铝反应时,这涉及消除一个烷基。配合物 5 和 6 是通过 1,2-苯基桥二胺 (L3) 与 AlEt3 在甲苯或四氢呋喃中反应合成的。值得注意的是,与其他配体相比,L3 表现出独特的反应性,当与烷基铝反应时形成三齿四配位结构。三齿复合物的形成是由于苯并咪唑衍生物或四氢呋喃 (THF) 分子的引入以及在与烷基铝配位过程中两个烷基的消除。通过 1H NMR、13C NMR 和元素分析对所有复合物进行表征,并通过 X 射线确认结构测定。此外,以这些配合物为催化剂,研究了醛、酮和亚胺在硼氢化反应中的催化活性。 其中,复合物 1 在温和的反应条件下,在低催化剂负载量 (1 mol%) 下表现出优异的催化性能(高达 99% 的产率)和广泛的底物相容性(超过 30 种底物)。
更新日期:2024-10-03
中文翻译:

烷基铝配合物,具有桥式双甲酰基芴酰亚胺配体,用于醛、酮和亚胺的氢硼化反应
设计合成了三种具有不同桥接基团的双甲酰基芴酰亚胺配体,通过与烷基铝试剂 (AlMe3 或 AlEt3) 反应成功制备了 6 种新型烷基铝配合物。通过 1,2-丙烯桥二胺 (L1) 与 AlMe3 或 AlEt3 反应获得复合物 1 和 2。通过将 1,2-环己基桥二胺 (L2) 与 AlMe3 或 AlEt3 反应得到络合物 3 和 4。上述配体与烷基铝形成双齿四配位结构,当配体与烷基铝反应时,这涉及消除一个烷基。配合物 5 和 6 是通过 1,2-苯基桥二胺 (L3) 与 AlEt3 在甲苯或四氢呋喃中反应合成的。值得注意的是,与其他配体相比,L3 表现出独特的反应性,当与烷基铝反应时形成三齿四配位结构。三齿复合物的形成是由于苯并咪唑衍生物或四氢呋喃 (THF) 分子的引入以及在与烷基铝配位过程中两个烷基的消除。通过 1H NMR、13C NMR 和元素分析对所有复合物进行表征,并通过 X 射线确认结构测定。此外,以这些配合物为催化剂,研究了醛、酮和亚胺在硼氢化反应中的催化活性。 其中,复合物 1 在温和的反应条件下,在低催化剂负载量 (1 mol%) 下表现出优异的催化性能(高达 99% 的产率)和广泛的底物相容性(超过 30 种底物)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号