当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Functionalization of Alkenes and Alkynes by Dinuclear Manganese Catalysts
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-10-02 , DOI: 10.1021/acs.accounts.4c00385 Fei Wang, Guichao Dong, Suqi Yang, Cheng-Long Ji, Kai Liu, Jie Han, Jin Xie
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-10-02 , DOI: 10.1021/acs.accounts.4c00385 Fei Wang, Guichao Dong, Suqi Yang, Cheng-Long Ji, Kai Liu, Jie Han, Jin Xie
|
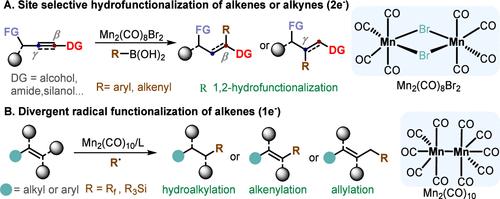
Alkenes and alkynes are fundamental building blocks in organic synthesis due to their commercial availability, bench-stability, and easy preparation. Selective functionalization of alkenes and alkynes is a crucial step for the synthesis of value-added compounds. Precise control over these reactions allows efficient construction of complex molecules with new functionalities. In recent decades, second- and third-row precious transition metal catalysts (palladium, platinum, rhodium, ruthenium) have been pivotal in the development of metal-catalyzed synthetic methodology. These metals exhibit excellent catalytic activity and selectivity, enabling efficient synthesis of functionalized organic molecules. However, recovery and reuse of precious metals have long been a challenge in this field. In recent years, exploration of earth-abundant metal-catalyzed organic reactions has interested both academic and industrial researchers. The development of such catalytic systems offers a promising approach to overcome the limitations of precious metal catalysts. For example, manganese is the third most naturally abundant transition metal with minimal toxicity and excellent biocompatibility. It exhibits good catalytic activity in several organic reactions, including C–H bond functionalization, selective reduction, and radical reactions. This Account outlines our recent progress in dinuclear manganese catalysis for selective functionalization of alkenes and alkynes. We have established the elementary manganese(I)-catalysis in transmetalation with R-B(OH)2. This finding has enabled us to apply the catalyst for the selective 1,2-difunctionalization of structurally diverse alkenes and alkynes. Mechanistic studies suggest a double manganese center synergistic activation model, as superior to Mn(CO)5Br in some cases. In addition, we have developed a ligand-tuned metalloradical strategy of dinuclear manganese catalysts (Mn2(CO)10), bridging the gap between the organometallics and radical chemistry, highlighting the unique radical functionalization of alkenes. Interestingly, using the same starting materials, different ligands can deliver completely different products. Meanwhile, a cooperative catalysis strategy involving manganese and other catalysts (e.g., cobalt, iminium) has also been developed and is briefly discussed. For manganese/iminium synergistic catalysis, a new mechanism for migratory insertion and demetalization–isomerization in synergistic HOMO–LUMO activation was disclosed. This strategy expands the application of low-valent manganese catalysts for enantioselective C–C bond-forming reactions. New reaction discovery is outpacing mechanism studies for dinuclear manganese catalysis, and future studies with time-resolved spectroscopy will improve understanding of the mechanism. Based on these intriguing findings, the precise functionalization of alkenes and alkynes by dinuclear manganese catalysts will expedite a novel activation model to enable late-stage functionalization of complex molecules.
中文翻译:

二核锰催化剂对烯烃和炔烃的选择性官能化
烯烃和炔烃是有机合成的基本组成部分,因为它们具有商业可用性、工作台稳定性和易于制备等优点。烯烃和炔烃的选择性官能化是合成高附加值化合物的关键步骤。对这些反应的精确控制可以有效地构建具有新功能的复杂分子。近几十年来,第二排和第三排贵金属过渡金属催化剂(钯、铂、铑、钌)在金属催化合成方法的开发中发挥了关键作用。这些金属表现出优异的催化活性和选择性,能够高效合成功能化有机分子。然而,贵金属的回收和再利用长期以来一直是该领域的一个挑战。近年来,对地球上丰富的金属催化有机反应的探索引起了学术和工业研究人员的兴趣。这种催化系统的开发为克服贵金属催化剂的局限性提供了一种有前途的方法。例如,锰是天然含量第三高的过渡金属,具有最小的毒性和出色的生物相容性。它在多种有机反应中表现出良好的催化活性,包括 C-H 键功能化、选择性还原和自由基反应。本报告概述了我们在二核锰催化烯烃和炔烃选择性官能化方面的最新进展。我们已经建立了用 R-B(OH)2 进行金属转移反应中的基本锰 (I) 催化。这一发现使我们能够将催化剂应用于结构多样的烯烃和炔烃的选择性 1,2-二官能化。 机理研究表明,双锰中心协同激活模型,在某些情况下优于 Mn(CO)5Br。此外,我们还开发了一种二核锰催化剂 (Mn2(CO)10) 的配体调整金属自由基策略,弥合了有机金属化合物和自由基化学之间的差距,突出了烯烃独特的自由基官能团化。有趣的是,使用相同的起始材料,不同的配体可以产生完全不同的产物。同时,还开发了一种涉及锰和其他催化剂(例如钴、亚胺)的协同催化策略,并进行了简要讨论。对于锰/亚胺协同催化,揭示了协同 HOMO-LUMO 活化中迁移插入和脱金属-异构化的新机制。该策略扩展了低价锰催化剂在对映选择性 C-C 键形成反应中的应用。新的反应发现超过了二核锰催化的机理研究,未来对时间分辨光谱的研究将提高对机理的理解。基于这些有趣的发现,二核锰催化剂对烯烃和炔烃的精确官能化将加速一种新的活化模型,以实现复杂分子的后期官能化。
更新日期:2024-10-02
中文翻译:

二核锰催化剂对烯烃和炔烃的选择性官能化
烯烃和炔烃是有机合成的基本组成部分,因为它们具有商业可用性、工作台稳定性和易于制备等优点。烯烃和炔烃的选择性官能化是合成高附加值化合物的关键步骤。对这些反应的精确控制可以有效地构建具有新功能的复杂分子。近几十年来,第二排和第三排贵金属过渡金属催化剂(钯、铂、铑、钌)在金属催化合成方法的开发中发挥了关键作用。这些金属表现出优异的催化活性和选择性,能够高效合成功能化有机分子。然而,贵金属的回收和再利用长期以来一直是该领域的一个挑战。近年来,对地球上丰富的金属催化有机反应的探索引起了学术和工业研究人员的兴趣。这种催化系统的开发为克服贵金属催化剂的局限性提供了一种有前途的方法。例如,锰是天然含量第三高的过渡金属,具有最小的毒性和出色的生物相容性。它在多种有机反应中表现出良好的催化活性,包括 C-H 键功能化、选择性还原和自由基反应。本报告概述了我们在二核锰催化烯烃和炔烃选择性官能化方面的最新进展。我们已经建立了用 R-B(OH)2 进行金属转移反应中的基本锰 (I) 催化。这一发现使我们能够将催化剂应用于结构多样的烯烃和炔烃的选择性 1,2-二官能化。 机理研究表明,双锰中心协同激活模型,在某些情况下优于 Mn(CO)5Br。此外,我们还开发了一种二核锰催化剂 (Mn2(CO)10) 的配体调整金属自由基策略,弥合了有机金属化合物和自由基化学之间的差距,突出了烯烃独特的自由基官能团化。有趣的是,使用相同的起始材料,不同的配体可以产生完全不同的产物。同时,还开发了一种涉及锰和其他催化剂(例如钴、亚胺)的协同催化策略,并进行了简要讨论。对于锰/亚胺协同催化,揭示了协同 HOMO-LUMO 活化中迁移插入和脱金属-异构化的新机制。该策略扩展了低价锰催化剂在对映选择性 C-C 键形成反应中的应用。新的反应发现超过了二核锰催化的机理研究,未来对时间分辨光谱的研究将提高对机理的理解。基于这些有趣的发现,二核锰催化剂对烯烃和炔烃的精确官能化将加速一种新的活化模型,以实现复杂分子的后期官能化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号