Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
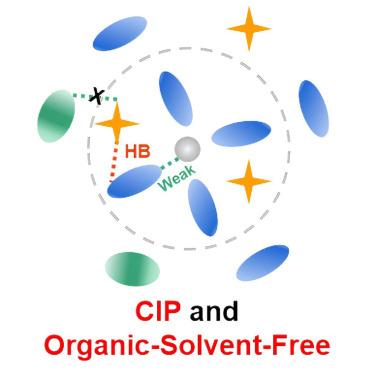
Organic-solvent-free primary solvation shell for low-temperature aqueous zinc batteries
Chem ( IF 19.1 ) Pub Date : 2024-10-02 , DOI: 10.1016/j.chempr.2024.09.001 Lishan Geng, Jiashen Meng, Xuanpeng Wang, Weidong Wu, Kang Han, Meng Huang, Chunhua Han, Lu Wu, Jinghao Li, Liang Zhou, Liqiang Mai
Chem ( IF 19.1 ) Pub Date : 2024-10-02 , DOI: 10.1016/j.chempr.2024.09.001 Lishan Geng, Jiashen Meng, Xuanpeng Wang, Weidong Wu, Kang Han, Meng Huang, Chunhua Han, Lu Wu, Jinghao Li, Liang Zhou, Liqiang Mai
|
Conventional hybrid aqueous electrolytes with solvated organic co-solvents encounter sluggish desolvation kinetics, especially under low-temperature conditions, due to the strong binding of organic solvents with Zn2+. Here, we develop a class of hybrid aqueous electrolytes with an organic-solvent-free primary solvation shell, favoring facile desolvation. As demonstrated by 1 M zinc acetate with dimethyl sulfoxide (DMSO) dipolar aprotic solvent, CH3COO− and H2O surround Zn2+, forming Zn2+(CH3COO−)2(H2O)4 clusters. The enhanced hydrogen bonds between solvated CH3COO− and H2O hinder DMSO from replacing solvated H2O. This weak solvation structure facilitates fast charge transfer kinetics and rapid Zn2+ flow through gradient solid electrolyte interphase. At −20°C, stable plating/stripping (5,600 h) and high Zn utilization (51%) are achieved. Furthermore, polyaniline||Zn batteries manifest low polarization (0.05 V), long cycling (8,800 cycles), and high rate. Importantly, this design strategy is generally extended to other hybrid electrolyte systems. This work represents advancements in electrolyte design for aqueous batteries.
中文翻译:

用于低温水性锌电池的无有机溶剂初级溶剂化壳
由于有机溶剂与 Zn2+ 的强结合,具有溶剂化有机助溶剂的常规杂化水性电解质会遇到缓慢的脱溶剂化动力学,尤其是在低温条件下。在这里,我们开发了一类具有无有机溶剂初级溶剂溶剂化壳层的杂化水性电解质,有利于简单的脱溶剂。如1 M乙酸锌与二甲基亚砜(DMSO)偶极非质子溶剂所示,CH3COO − 和 H2O 围绕Zn2 +,形成Zn2 +(CH3COO - )2(H2O)4簇。溶剂化 CH3COO− 和 H2O 之间增强的氢键阻碍了 DMSO 取代溶剂化的 H2O。这种弱溶剂化结构有助于快速电荷转移动力学和 Zn2+ 快速流过梯度固体电解质界面。在 −20°C 时,可实现稳定的电镀/剥离 (5,600 h) 和高 Zn 利用率 (51%)。此外,聚苯胺||锌电池具有低极化 (0.05 V)、长循环 (8,800 次循环) 和高倍率等特点。重要的是,这种设计策略通常扩展到其他混合电解质系统。这项工作代表了水性电池电解质设计的进步。
更新日期:2024-10-02
中文翻译:

用于低温水性锌电池的无有机溶剂初级溶剂化壳
由于有机溶剂与 Zn2+ 的强结合,具有溶剂化有机助溶剂的常规杂化水性电解质会遇到缓慢的脱溶剂化动力学,尤其是在低温条件下。在这里,我们开发了一类具有无有机溶剂初级溶剂溶剂化壳层的杂化水性电解质,有利于简单的脱溶剂。如1 M乙酸锌与二甲基亚砜(DMSO)偶极非质子溶剂所示,CH3COO − 和 H2O 围绕Zn2 +,形成Zn2 +(CH3COO - )2(H2O)4簇。溶剂化 CH3COO− 和 H2O 之间增强的氢键阻碍了 DMSO 取代溶剂化的 H2O。这种弱溶剂化结构有助于快速电荷转移动力学和 Zn2+ 快速流过梯度固体电解质界面。在 −20°C 时,可实现稳定的电镀/剥离 (5,600 h) 和高 Zn 利用率 (51%)。此外,聚苯胺||锌电池具有低极化 (0.05 V)、长循环 (8,800 次循环) 和高倍率等特点。重要的是,这种设计策略通常扩展到其他混合电解质系统。这项工作代表了水性电池电解质设计的进步。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号