Diabetologia ( IF 8.4 ) Pub Date : 2024-10-01 , DOI: 10.1007/s00125-024-06274-6 Lue Ping Zhao, George K. Papadopoulos, Jay S. Skyler, Alberto Pugliese, Hemang M. Parikh, William W. Kwok, Terry P. Lybrand, George P. Bondinas, Antonis K. Moustakas, Ruihan Wang, Chul-Woo Pyo, Wyatt C. Nelson, Daniel E. Geraghty, Åke Lernmark
|
Aims/hypothesis
The aim of this work was to explore molecular amino acids (AAs) and related structures of HLA-DQA1-DQB1 that underlie its contribution to the progression from stages 1 or 2 to stage 3 type 1 diabetes.
Methods
Using high-resolution DQA1 and DQB1 genotypes from 1216 participants in the Diabetes Prevention Trial-Type 1 and the Diabetes Prevention Trial, we applied hierarchically organised haplotype association analysis (HOH) to decipher which AAs contributed to the associations of DQ with disease and their structural properties. HOH relied on the Cox regression to quantify the association of DQ with time-to-onset of type 1 diabetes.
Results
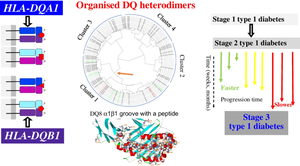
By numerating all possible DQ heterodimers of α- and β-chains, we showed that the heterodimerisation increases genetic diversity at the cellular level from 43 empirically observed haplotypes to 186 possible heterodimers. Heterodimerisation turned several neutral haplotypes (DQ2.2, DQ2.3 and DQ4.4) to risk haplotypes (DQ2.2/2.3-DQ4.4 and DQ4.4-DQ2.2). HOH uncovered eight AAs on the α-chain (−16α, −13α, −6α, α22, α23, α44, α72, α157) and six AAs on the β-chain (−18β, β9, β13, β26, β57, β135) that contributed to the association of DQ with progression of type 1 diabetes. The specific AAs concerned the signal peptide (minus sign, possible linkage to expression levels), pockets 1, 4 and 9 in the antigen-binding groove of the α1β1 domain, and the putative homodimerisation of the αβ heterodimers.
Conclusions/interpretation
These results unveil the contribution made by DQ to type 1 diabetes progression at individual residues and related protein structures, shedding light on its immunological mechanisms and providing new leads for developing treatment strategies.
Data availability
Clinical trial data and biospecimen samples are available through the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository portal (https://repository.niddk.nih.gov/studies).
Graphical Abstract
中文翻译:

在 DPT-1 和 TN07 临床试验中,进展为 1 型糖尿病与 HLA-DQA1-B1 异二聚体中的特定残基密切相关
目标/假设
这项工作的目的是探索 HLA-DQA1-DQB1 的分子氨基酸 (AAs) 和相关结构,这些结构是其对 1 期或 2 期向 3 期 1 型糖尿病进展的贡献的基础。
方法
使用来自 1 型糖尿病预防试验和糖尿病预防试验的 1216 名参与者的高分辨率 DQA1 和 DQB1 基因型,我们应用分层组织的单倍型关联分析 (HOH) 来破译哪些 AA 有助于 DQ 与疾病及其结构特性的关联。HOH 依靠 Cox 回归来量化 DQ 与 1 型糖尿病发病时间的相关性。
结果
通过对 α 链和 β 链的所有可能的 DQ 异二聚体进行计数,我们表明异二聚化将细胞水平的遗传多样性从经验观察到的 43 个单倍型增加到 186 个可能的异二聚体。异二聚化将几种中性单倍型 (DQ2.2 、 DQ2.3 和 DQ4.4) 转变为风险单倍型 (DQ2.2/2.3-DQ4.4 和 DQ4.4-DQ2.2)。HOH 发现 α 链上有 8 个 AAs (-16α, -13α, -6α, α22, α23, α44, α72, α157) 和 β链上有 6 个 AAs (-18β, β9, β13, β26, β57, β135),这些 AAs 导致 DQ 与 1 型糖尿病进展相关。特异性 AA 涉及信号肽 (减号,可能与表达水平相关)、α1β1 结构域抗原结合槽中的口袋 1、4 和 9,以及 αβ 异二聚体的推定同源二聚化。
结论/解释
这些结果揭示了 DQ 对单个残基和相关蛋白质结构对 1 型糖尿病进展的贡献,阐明了其免疫机制,并为开发治疗策略提供了新的线索。
数据可用性
临床试验数据和生物样本样本可通过美国国家糖尿病、消化和肾脏疾病研究所中央存储库门户 (https://repository.niddk.nih.gov/studies) 获得。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号