当前位置:
X-MOL 学术
›
Eur. J. Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long‐term follow‐up of the TRED‐HF trial: Implications for therapy in patients with dilated cardiomyopathy and heart failure remission
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-10-01 , DOI: 10.1002/ejhf.3475 Leanne Cheng, Daniel Hammersley, Aaraby Ragavan, Saad Javed, Srinjay Mukhopadhyay, John Gregson, Jennie Han, Zohya Khalique, Amrit Lota, Antonis Pantazis, A. John Baksi, Gerald Carr‐White, Antonio de Marvao, James Ware, Upasana Tayal, Dudley J. Pennell, John G.F. Cleland, Sanjay K. Prasad, Brian P. Halliday
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-10-01 , DOI: 10.1002/ejhf.3475 Leanne Cheng, Daniel Hammersley, Aaraby Ragavan, Saad Javed, Srinjay Mukhopadhyay, John Gregson, Jennie Han, Zohya Khalique, Amrit Lota, Antonis Pantazis, A. John Baksi, Gerald Carr‐White, Antonio de Marvao, James Ware, Upasana Tayal, Dudley J. Pennell, John G.F. Cleland, Sanjay K. Prasad, Brian P. Halliday
|
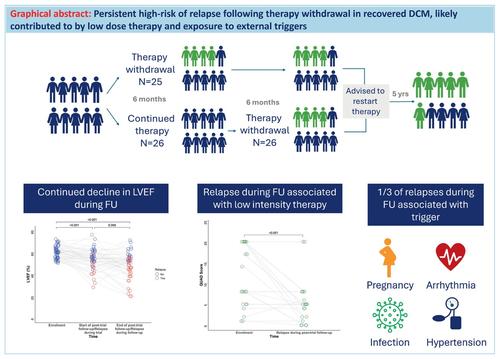
AimsIn TRED‐HF, 40% of patients with recovered dilated cardiomyopathy (DCM) relapsed in the short term after therapy withdrawal. This follow‐up investigates the longer‐term effects of therapy withdrawal.Methods and resultsTRED‐HF was a randomized trial investigating heart failure therapy withdrawal in patients with recovered DCM over 6 months. Those randomized to continue therapy subsequently withdrew treatment between 6 and 12 months. Participants were recommended to restart therapy post‐trial and were followed until May 2023. Clinical outcomes are reported in a non‐randomized fashion from enrolment and from the end of the trial. The primary outcome was relapse defined as ≥10% reduction in left ventricular ejection fraction to <50%, doubling in N‐terminal pro‐B‐type natriuretic peptide to >400 ng/L, or clinical features of heart failure. From enrolment to the last follow‐up (median 6 years, interquartile range 6–7), 33 of 51 patients (65%) relapsed. The 5‐year relapse rate from enrolment was 61% (95% confidence interval [CI] 45–73) and from the end of the trial was 39% (95% CI 19–54). Of 20 patients who relapsed during the trial, nine had a recurrent relapse during follow‐up. Thirteen relapsed for the first time after the trial; seven had restarted low intensity therapy, four had not restarted therapy and two did not have therapy withdrawn. The mean intensity of therapy was lower after the trial compared to enrolment (mean difference −6 [−8 to −4]; p < 0.001). One third of relapses during follow‐up had identifiable triggers (arrhythmia [n = 4], pregnancy [n = 1], hypertension [n = 1], infection [n = 1]). Corrected atrial fibrillation was associated with reduced risk of relapse (hazard ratio 0.33, 95% CI 0.12–0.96; p = 0.042).ConclusionsThe risk of relapse in the 5 years following the TRED‐HF trial remained high. Restarting lower doses of heart failure medications at the end of the trial, external triggers and disease progression are likely to have contributed to relapse.
中文翻译:

TRED-HF 试验的长期随访:对扩张型心肌病和心力衰竭缓解患者的治疗意义
目标在 TRED-HF 中,40% 恢复的扩张型心肌病 (DCM) 患者在停药后短期内复发。本次随访调查了停止治疗的长期影响。方法和结果TRED-HF 是一项随机试验,调查 DCM 恢复 6 个月以上患者的心力衰竭治疗停止情况。那些随机继续治疗的患者随后在 6 至 12 个月之间退出治疗。建议参与者在试验后重新开始治疗,并随访至 2023 年 5 月。从入组到试验结束,以非随机方式报告临床结果。主要结局是复发,定义为左心室射血分数降低≥10%至<50%,N末端B型利钠肽前体加倍至>400 ng/L,或心力衰竭的临床特征。从入组到最后一次随访(中位时间 6 年,四分位数范围 6-7),51 名患者中有 33 名 (65%) 复发。入组后 5 年复发率为 61%(95% 置信区间 [CI] 45-73),试验结束后 5 年复发率为 39%(95% CI 19-54)。在试验期间复发的 20 名患者中,有 9 名在随访期间复发。审判后首次复发有13人;七人重新开始低强度治疗,四人没有重新开始治疗,两人没有撤回治疗。与入组相比,试验后的平均治疗强度较低(平均差 -6 [-8 至 -4];p < 0.001)。随访期间三分之一的复发有可识别的触发因素(心律失常 [n = 4]、妊娠 [n = 1]、高血压 [n = 1]、感染 [n = 1])。房颤纠正与复发风险降低相关(风险比 0.33,95% CI 0.12–0.96;p = 0.042)。结论 TRED-HF 试验后 5 年内复发的风险仍然很高。在试验结束时重新开始较低剂量的心力衰竭药物、外部触发因素和疾病进展可能导致复发。
更新日期:2024-10-01
中文翻译:

TRED-HF 试验的长期随访:对扩张型心肌病和心力衰竭缓解患者的治疗意义
目标在 TRED-HF 中,40% 恢复的扩张型心肌病 (DCM) 患者在停药后短期内复发。本次随访调查了停止治疗的长期影响。方法和结果TRED-HF 是一项随机试验,调查 DCM 恢复 6 个月以上患者的心力衰竭治疗停止情况。那些随机继续治疗的患者随后在 6 至 12 个月之间退出治疗。建议参与者在试验后重新开始治疗,并随访至 2023 年 5 月。从入组到试验结束,以非随机方式报告临床结果。主要结局是复发,定义为左心室射血分数降低≥10%至<50%,N末端B型利钠肽前体加倍至>400 ng/L,或心力衰竭的临床特征。从入组到最后一次随访(中位时间 6 年,四分位数范围 6-7),51 名患者中有 33 名 (65%) 复发。入组后 5 年复发率为 61%(95% 置信区间 [CI] 45-73),试验结束后 5 年复发率为 39%(95% CI 19-54)。在试验期间复发的 20 名患者中,有 9 名在随访期间复发。审判后首次复发有13人;七人重新开始低强度治疗,四人没有重新开始治疗,两人没有撤回治疗。与入组相比,试验后的平均治疗强度较低(平均差 -6 [-8 至 -4];p < 0.001)。随访期间三分之一的复发有可识别的触发因素(心律失常 [n = 4]、妊娠 [n = 1]、高血压 [n = 1]、感染 [n = 1])。房颤纠正与复发风险降低相关(风险比 0.33,95% CI 0.12–0.96;p = 0.042)。结论 TRED-HF 试验后 5 年内复发的风险仍然很高。在试验结束时重新开始较低剂量的心力衰竭药物、外部触发因素和疾病进展可能导致复发。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号