当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluxional halogen bonds in linear complexes of tetrafluorodiiodobenzene with dinitrobenzene
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-10-01 , DOI: 10.1002/jcc.27483 Cai-Yue Gao, Bin-Bin Pei, Si-Dian Li
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-10-01 , DOI: 10.1002/jcc.27483 Cai-Yue Gao, Bin-Bin Pei, Si-Dian Li
|
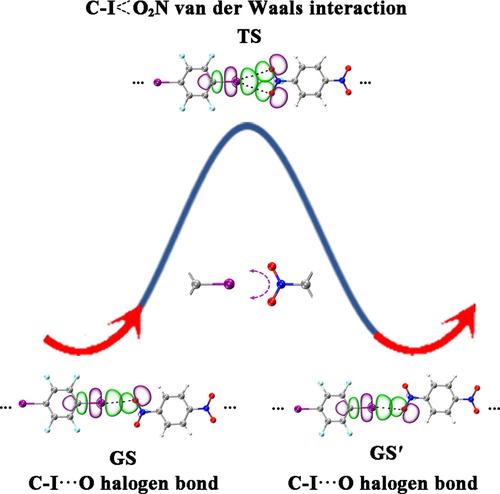
The fluxional nature of halogen bonds (XBs) in small molecular clusters, supramolecules, and molecular crystals has received considerable attention in recent years. In this work, based on extensive density-functional theory calculations and detailed electrostatic potential (ESP), natural bonding orbital (NBO), non-covalent interactions-reduced density gradient (NCI-RDG), and quantum theory of atoms in molecules (QTAIM) analyses, we unveil the existence of fluxional halogen bonds (FXBs) in a series of linear (IC6F4I)m(OONC6H4NOO)n (m + n = 2–5) complexes of tetrafluorodiiodobenzene with dinitrobenzene which appear to be similar to the previously reported fluxional hydrogen bonds (FHBs) in small water clusters (H2O)n (n = 2–6). The obtained  O2N van der Waals interaction in the transition state (TS). The cohesive energies (Ecoh) of these complexes with up to four XBs exhibit an almost perfect linear relationship with the numbers of XBs in the systems, with the average calculated halogen bond energy of Ecoh/XB = 3.48 kcal·mol−1 in the ground states which appears to be about 55% of the average calculated hydrogen bond energy (Ecoh/HB = 6.28 kcal·mol−1) in small water clusters.
O2N van der Waals interaction in the transition state (TS). The cohesive energies (Ecoh) of these complexes with up to four XBs exhibit an almost perfect linear relationship with the numbers of XBs in the systems, with the average calculated halogen bond energy of Ecoh/XB = 3.48 kcal·mol−1 in the ground states which appears to be about 55% of the average calculated hydrogen bond energy (Ecoh/HB = 6.28 kcal·mol−1) in small water clusters.
中文翻译:

四氟二碘苯与二硝基苯线性络合物中的助熔卤素键
近年来,小分子簇、超分子和分子晶体中卤素键 (XBs) 的通量性质受到了相当大的关注。在这项工作中,基于广泛的密度泛函理论计算和详细的静电势 (ESP)、自然键轨道 (NBO)、非共价相互作用-还原密度梯度 (NCI-RDG) 和分子中原子的量子理论 (QTAIM) 分析,我们揭示了通量卤素键 (FXB) 在一系列线性 (IC6F4I)m(OONC6H4NOO)n (m + n= 2-5) 四氟二碘苯与二硝基苯的络合物,其似乎类似于先前报道的小水簇 (H2O)n (n = 2-6) 中的通量氢键 (FHB)。得到的 磁通机制涉及系统中的一个 FXB,该 FXB 在两个线性 CI···基态中的 O XB(GS 和 GS')通过过渡态 (TS) 中的分叉 CI  O2N 范德华相互作用。这些具有多达四个 XB 的配合物的内聚能 (Ecoh) 与系统中 XB 的数量表现出几乎完美的线性关系,在基态下,E coh/XB = 3.48 kcal·mol-1 的平均计算卤素键能似乎约为平均计算的氢键能 (Ecoh/HB = 6.28 kcal·mol-1) 的 55% 在小水团簇中。
O2N 范德华相互作用。这些具有多达四个 XB 的配合物的内聚能 (Ecoh) 与系统中 XB 的数量表现出几乎完美的线性关系,在基态下,E coh/XB = 3.48 kcal·mol-1 的平均计算卤素键能似乎约为平均计算的氢键能 (Ecoh/HB = 6.28 kcal·mol-1) 的 55% 在小水团簇中。
更新日期:2024-10-01
 O2N van der Waals interaction in the transition state (TS). The cohesive energies (Ecoh) of these complexes with up to four XBs exhibit an almost perfect linear relationship with the numbers of XBs in the systems, with the average calculated halogen bond energy of Ecoh/XB = 3.48 kcal·mol−1 in the ground states which appears to be about 55% of the average calculated hydrogen bond energy (Ecoh/HB = 6.28 kcal·mol−1) in small water clusters.
O2N van der Waals interaction in the transition state (TS). The cohesive energies (Ecoh) of these complexes with up to four XBs exhibit an almost perfect linear relationship with the numbers of XBs in the systems, with the average calculated halogen bond energy of Ecoh/XB = 3.48 kcal·mol−1 in the ground states which appears to be about 55% of the average calculated hydrogen bond energy (Ecoh/HB = 6.28 kcal·mol−1) in small water clusters.
中文翻译:

四氟二碘苯与二硝基苯线性络合物中的助熔卤素键
近年来,小分子簇、超分子和分子晶体中卤素键 (XBs) 的通量性质受到了相当大的关注。在这项工作中,基于广泛的密度泛函理论计算和详细的静电势 (ESP)、自然键轨道 (NBO)、非共价相互作用-还原密度梯度 (NCI-RDG) 和分子中原子的量子理论 (QTAIM) 分析,我们揭示了通量卤素键 (FXB) 在一系列线性 (IC6F4I)m(OONC6H4NOO)n (m + n= 2-5) 四氟二碘苯与二硝基苯的络合物,其似乎类似于先前报道的小水簇 (H2O)n (n = 2-6) 中的通量氢键 (FHB)。得到
 O2N 范德华相互作用。这些具有多达四个 XB 的配合物的内聚能 (Ecoh) 与系统中 XB 的数量表现出几乎完美的线性关系,在基态下,E coh/XB = 3.48 kcal·mol-1 的平均计算卤素键能似乎约为平均计算的氢键能 (Ecoh/HB = 6.28 kcal·mol-1) 的 55% 在小水团簇中。
O2N 范德华相互作用。这些具有多达四个 XB 的配合物的内聚能 (Ecoh) 与系统中 XB 的数量表现出几乎完美的线性关系,在基态下,E coh/XB = 3.48 kcal·mol-1 的平均计算卤素键能似乎约为平均计算的氢键能 (Ecoh/HB = 6.28 kcal·mol-1) 的 55% 在小水团簇中。































 京公网安备 11010802027423号
京公网安备 11010802027423号