Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antiviral innate immune memory in alveolar macrophages following SARS-CoV-2 infection ameliorates secondary influenza A virus disease
Immunity ( IF 25.5 ) Pub Date : 2024-09-30 , DOI: 10.1016/j.immuni.2024.08.018 Alexander Lercher, Jin-Gyu Cheong, Michael J. Bale, Chenyang Jiang, Hans-Heinrich Hoffmann, Alison W. Ashbrook, Tyler Lewy, Yue S. Yin, Corrine Quirk, Emma J. DeGrace, Luis Chiriboga, Brad R. Rosenberg, Steven Z. Josefowicz, Charles M. Rice
Immunity ( IF 25.5 ) Pub Date : 2024-09-30 , DOI: 10.1016/j.immuni.2024.08.018 Alexander Lercher, Jin-Gyu Cheong, Michael J. Bale, Chenyang Jiang, Hans-Heinrich Hoffmann, Alison W. Ashbrook, Tyler Lewy, Yue S. Yin, Corrine Quirk, Emma J. DeGrace, Luis Chiriboga, Brad R. Rosenberg, Steven Z. Josefowicz, Charles M. Rice
|
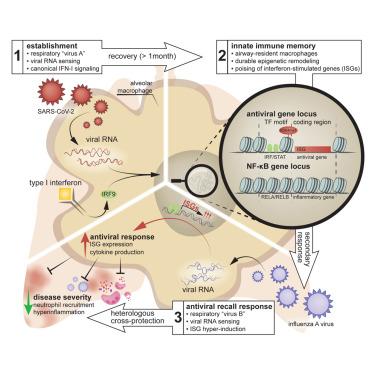
Pathogen encounter can result in epigenetic remodeling that shapes disease caused by heterologous pathogens. Here, we examined innate immune memory in the context of commonly circulating respiratory viruses. Single-cell analyses of airway-resident immune cells in a disease-relevant murine model of SARS-CoV-2 recovery revealed epigenetic reprogramming in alveolar macrophages following infection. Post-COVID-19 human monocytes exhibited similar epigenetic signatures. In airway-resident macrophages, past SARS-CoV-2 infection increased activity of type I interferon (IFN-I)-related transcription factors and epigenetic poising of antiviral genes. Viral pattern recognition and canonical IFN-I signaling were required for the establishment of this innate immune memory and augmented secondary antiviral responses. Antiviral innate immune memory mounted by airway-resident macrophages post-SARS-CoV-2 was necessary and sufficient to ameliorate secondary disease caused by influenza A virus and curtailed hyperinflammatory dysregulation and mortality. Our findings provide insights into antiviral innate immune memory in the airway that may facilitate the development of broadly effective therapeutic strategies.
中文翻译:

SARS-CoV-2 感染后肺泡巨噬细胞的抗病毒先天免疫记忆可改善继发性甲型流感病毒病
病原体的遭遇可导致表观遗传重塑,从而塑造由异源病原体引起的疾病。在这里,我们在常见传播的呼吸道病毒的背景下检查了先天免疫记忆。在 SARS-CoV-2 恢复的疾病相关小鼠模型中对气道驻留免疫细胞的单细胞分析揭示了感染后肺泡巨噬细胞的表观遗传重编程。COVID-19 后人类单核细胞表现出相似的表观遗传特征。在气道驻留巨噬细胞中,过去的 SARS-CoV-2 感染增加了 I 型干扰素 (IFN-I) 相关转录因子的活性和抗病毒基因的表观遗传平衡。病毒模式识别和经典 IFN-I 信号转导是建立这种先天免疫记忆和增强继发性抗病毒反应所必需的。SARS-CoV-2 后气道驻留巨噬细胞所携带的抗病毒先天免疫记忆对于改善甲型流感病毒引起的继发性疾病和减少过度炎症失调和死亡率是必要且足够的。我们的研究结果提供了对气道中抗病毒先天免疫记忆的见解,这可能有助于开发广泛有效的治疗策略。
更新日期:2024-09-30
中文翻译:

SARS-CoV-2 感染后肺泡巨噬细胞的抗病毒先天免疫记忆可改善继发性甲型流感病毒病
病原体的遭遇可导致表观遗传重塑,从而塑造由异源病原体引起的疾病。在这里,我们在常见传播的呼吸道病毒的背景下检查了先天免疫记忆。在 SARS-CoV-2 恢复的疾病相关小鼠模型中对气道驻留免疫细胞的单细胞分析揭示了感染后肺泡巨噬细胞的表观遗传重编程。COVID-19 后人类单核细胞表现出相似的表观遗传特征。在气道驻留巨噬细胞中,过去的 SARS-CoV-2 感染增加了 I 型干扰素 (IFN-I) 相关转录因子的活性和抗病毒基因的表观遗传平衡。病毒模式识别和经典 IFN-I 信号转导是建立这种先天免疫记忆和增强继发性抗病毒反应所必需的。SARS-CoV-2 后气道驻留巨噬细胞所携带的抗病毒先天免疫记忆对于改善甲型流感病毒引起的继发性疾病和减少过度炎症失调和死亡率是必要且足够的。我们的研究结果提供了对气道中抗病毒先天免疫记忆的见解,这可能有助于开发广泛有效的治疗策略。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号