当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-catalyzed enantioselective hydroetherification of alkenes and symmetric 1,3-diketones
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-01 , DOI: 10.1016/j.checat.2024.101126 Meihui Guan, Lihan Zhu, Yue Wang, Ge Zhang, Huanran Miao, Bei Chen, Qian Zhang
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-01 , DOI: 10.1016/j.checat.2024.101126 Meihui Guan, Lihan Zhu, Yue Wang, Ge Zhang, Huanran Miao, Bei Chen, Qian Zhang
|
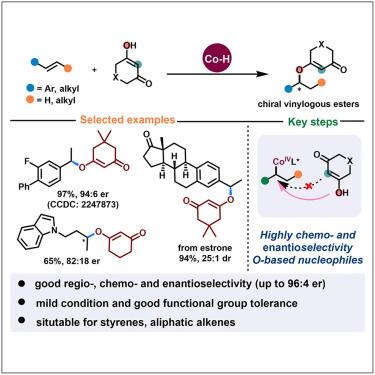
Catalytic asymmetric hydroetherification of alkenes constitutes an efficient strategy toward enantioenriched oxygenated building blocks from readily available starting materials. However, the enantioselective intermolecular transformation of simple alkenes is particularly underdeveloped. Here, a Co(III)-hydride-mediated enantioselective olefin hydroetherification through radical-polar crossover H atom transfer has been described, with cyclic 1,3-diketone derivatives as O nucleophilic partners. This practical method is applicable for both styrenes and aliphatic alkenes with good functional group tolerance, enabling facile access to structurally diverse chiral vinylogous ester derivatives with excellent regio-, chemo-, and enantioselectivity. Theoretical studies have shown that the formation of alkyl Co(III) intermediates and the SN2-substitution of alkyl Co(IV) with nucleophiles have an effect on the stereoselectivity of the products. Additionally, the O–H···π interaction between the –OH moiety of substrate moiety and salen ligand plays a crucial role in determining unique asymmetric C–O bond chemoselectivity compared to the disfavored steric hindrance in C–C bond construction.
中文翻译:

钴催化烯烃和对称 1,3-二酮的对映选择性加氢醚化
烯烃的催化不对称加氢醚化构成了从容易获得的起始材料制备对映体富集的含氧结构单元的有效策略。然而,简单烯烃的对映选择性分子间转化尤其不发达。在此,描述了通过自由基-极性交叉H原子转移的Co(III)-氢化物介导的对映选择性烯烃加氢醚化,其中环状1,3-二酮衍生物作为O亲核伙伴。这种实用的方法适用于苯乙烯和脂肪族烯烃,具有良好的官能团耐受性,能够轻松获得结构多样的手性插烯酯衍生物,并具有优异的区域选择性、化学选择性和对映选择性。理论研究表明,烷基Co(III)中间体的形成以及烷基Co(IV)与亲核试剂的S N 2-取代对产物的立体选择性有影响。此外,与 C-C 键构建中不利的空间位阻相比,底物部分的 -OH 部分与 salen 配体之间的 O-H·π 相互作用在确定独特的不对称 C-O 键化学选择性方面起着至关重要的作用。
更新日期:2024-10-01
中文翻译:

钴催化烯烃和对称 1,3-二酮的对映选择性加氢醚化
烯烃的催化不对称加氢醚化构成了从容易获得的起始材料制备对映体富集的含氧结构单元的有效策略。然而,简单烯烃的对映选择性分子间转化尤其不发达。在此,描述了通过自由基-极性交叉H原子转移的Co(III)-氢化物介导的对映选择性烯烃加氢醚化,其中环状1,3-二酮衍生物作为O亲核伙伴。这种实用的方法适用于苯乙烯和脂肪族烯烃,具有良好的官能团耐受性,能够轻松获得结构多样的手性插烯酯衍生物,并具有优异的区域选择性、化学选择性和对映选择性。理论研究表明,烷基Co(III)中间体的形成以及烷基Co(IV)与亲核试剂的S N 2-取代对产物的立体选择性有影响。此外,与 C-C 键构建中不利的空间位阻相比,底物部分的 -OH 部分与 salen 配体之间的 O-H·π 相互作用在确定独特的不对称 C-O 键化学选择性方面起着至关重要的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号