Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convergent synthesis and protein binding of vicinal difluorides by stereodivergent C–C bond formation
Chem ( IF 19.1 ) Pub Date : 2024-09-30 , DOI: 10.1016/j.chempr.2024.08.024 Yehao Qiu, Vienna C.J.X. Thomas, Tommaso Fantoni, Reichi Chen, Xingyu Jiang, Zhi-Tao He, Trevor W. Butcher, Daniel K. Nomura, John F. Hartwig
Chem ( IF 19.1 ) Pub Date : 2024-09-30 , DOI: 10.1016/j.chempr.2024.08.024 Yehao Qiu, Vienna C.J.X. Thomas, Tommaso Fantoni, Reichi Chen, Xingyu Jiang, Zhi-Tao He, Trevor W. Butcher, Daniel K. Nomura, John F. Hartwig
|
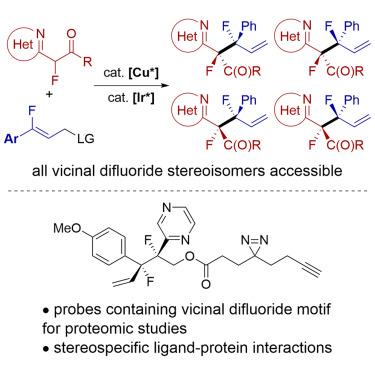
Vicinal difluorides adopt defined conformations due to the electronic properties of fluorine. Therefore, they could be valuable for controlling the constellation of functional groups about acyclic C–C bonds in organic molecules if all stereoisomers of the difluorides could be synthesized. However, stereoselective synthesis of vicinal difluorides has been cumbersome. The location of functional groups within organic molecules is important because it influences function, particularly biological function. We report a catalytic synthesis of acyclic vicinal difluoride stereoisomers by C–C bond formation between two monofluoro units, along with crystallographic and computational data showing that the gauche relationship of two fluorides causes substituents to occupy defined positions about the C(sp3)–C(sp3) bond. Photoreactive chemical probes tethered to vicinal difluorides showed that difluorides bind more strongly than the analogous monofluorides, which possess less defined conformations, and that individual stereoisomers of the difluorides bind distinctly to the human proteome.
中文翻译:

通过立体发散的 C-C 键形成连位二氟化物的聚合合成和蛋白质结合
由于氟的电子特性,邻位二氟化物采用确定的构象。因此,如果可以合成二氟化物的所有立体异构体,它们对于控制有机分子中无环 C-C 键的官能团群可能很有价值。然而,邻位二氟化物的立体选择性合成一直很麻烦。有机分子内官能团的位置很重要,因为它影响功能,特别是生物功能。我们报告了通过两个单氟单元之间形成 C-C 键来催化合成无环邻位二氟化物立体异构体,以及晶体学和计算数据,表明两种氟化物的稀疏关系导致取代基占据 C( sp 3 )-C 周围的定义位置( sp 3 ) 键。与邻位二氟化物连接的光反应化学探针表明,二氟化物比类似的一氟化物结合更牢固,后者具有不太确定的构象,并且二氟化物的各个立体异构体与人类蛋白质组明显结合。
更新日期:2024-10-01
中文翻译:

通过立体发散的 C-C 键形成连位二氟化物的聚合合成和蛋白质结合
由于氟的电子特性,邻位二氟化物采用确定的构象。因此,如果可以合成二氟化物的所有立体异构体,它们对于控制有机分子中无环 C-C 键的官能团群可能很有价值。然而,邻位二氟化物的立体选择性合成一直很麻烦。有机分子内官能团的位置很重要,因为它影响功能,特别是生物功能。我们报告了通过两个单氟单元之间形成 C-C 键来催化合成无环邻位二氟化物立体异构体,以及晶体学和计算数据,表明两种氟化物的稀疏关系导致取代基占据 C( sp 3 )-C 周围的定义位置( sp 3 ) 键。与邻位二氟化物连接的光反应化学探针表明,二氟化物比类似的一氟化物结合更牢固,后者具有不太确定的构象,并且二氟化物的各个立体异构体与人类蛋白质组明显结合。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号