当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Second-Coordination-Sphere Effects Reveal Electronic Structure Differences between the Mitochondrial Amidoxime Reducing Component and Sulfite Oxidase
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-30 , DOI: 10.1021/acs.inorgchem.4c02157 Michel A. Struwe, Jing Yang, Kubandiran Kolanji, Joshua Mengell, Axel J. Scheidig, Bernd Clement, Martin L. Kirk
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-30 , DOI: 10.1021/acs.inorgchem.4c02157 Michel A. Struwe, Jing Yang, Kubandiran Kolanji, Joshua Mengell, Axel J. Scheidig, Bernd Clement, Martin L. Kirk
|
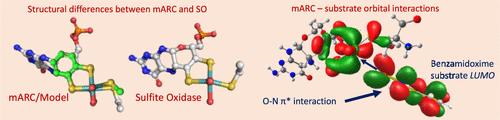
A combination of X-ray absorption and low-temperature electronic absorption spectroscopies has been used to probe the geometric and electronic structures of the human mitochondrial amidoxime reducing component enzyme (hmARC1) in the oxidized Mo(VI) and reduced Mo(IV) forms. Extended X-ray absorption fine structure analysis revealed that oxidized enzyme possesses a 5-coordinate [MoO2(SCys)(PDT)]− (PDT = pyranopterin dithiolene) active site with a cysteine coordinated to Mo. A 5-coordinate geometry is retained in the reduced state, with the equatorial oxo being protonated. Low-temperature electronic absorption spectroscopy of hmARC1 reveals a spectrum for the oxidized enzyme that is significantly different from what has been reported for sulfite oxidase family enzymes. Time-dependent density functional theory computations on oxidized and reduced hmARC1, and a small molecule analogue for hmARC1ox, have been used to assist us in making detailed band assignments and developing a greater understanding of enzyme electronic structure contributions to reactivity. Our understanding of the hmARCred HOMO and the LUMO of the benzamidoxime substrate reveal a potential π-bonding interaction between these redox orbitals, with two-electron occupation of the substrate LUMO along the reaction coordinate activating the O–N bond for cleavage and promoting oxygen atom transfer to the Mo site.
中文翻译:

第二配位球效应揭示了线粒体氨基肟还原组分和亚硫酸盐氧化酶之间的电子结构差异
X 射线吸收和低温电子吸收光谱的组合已被用于探测氧化 Mo(VI) 和还原 Mo(IV) 形式的人线粒体氨基肟还原成分酶 (hmARC1) 的几何和电子结构。扩展 X 射线吸收精细结构分析表明,氧化酶具有 5 配位 [MoO2(SCys)(PDT)]−(PDT = 吡喃蝶呤二硫醇)活性位点,其半胱氨酸与 Mo 配位。在还原状态下保留 5 坐标几何结构,赤道氧基被质子化。hmARC1 的低温电子吸收光谱揭示了氧化酶的光谱,该光谱与已报道的亚硫酸盐氧化酶家族酶的光谱显著不同。氧化和还原 hmARC1 的时间依赖性密度泛函理论计算以及 hmARC1ox 的小分子类似物已被用于帮助我们进行详细的条带分配,并更深入地了解酶电子结构对反应性的贡献。我们对苯扎米肟底物的 hmARCred HOMO 和 LUMO 的理解揭示了这些氧化还原轨道之间潜在的π键相互作用,底物 LUMO 沿反应坐标的双电子占据激活 O-N 键进行切割并促进氧原子转移到 Mo 位点。
更新日期:2024-09-30
中文翻译:

第二配位球效应揭示了线粒体氨基肟还原组分和亚硫酸盐氧化酶之间的电子结构差异
X 射线吸收和低温电子吸收光谱的组合已被用于探测氧化 Mo(VI) 和还原 Mo(IV) 形式的人线粒体氨基肟还原成分酶 (hmARC1) 的几何和电子结构。扩展 X 射线吸收精细结构分析表明,氧化酶具有 5 配位 [MoO2(SCys)(PDT)]−(PDT = 吡喃蝶呤二硫醇)活性位点,其半胱氨酸与 Mo 配位。在还原状态下保留 5 坐标几何结构,赤道氧基被质子化。hmARC1 的低温电子吸收光谱揭示了氧化酶的光谱,该光谱与已报道的亚硫酸盐氧化酶家族酶的光谱显著不同。氧化和还原 hmARC1 的时间依赖性密度泛函理论计算以及 hmARC1ox 的小分子类似物已被用于帮助我们进行详细的条带分配,并更深入地了解酶电子结构对反应性的贡献。我们对苯扎米肟底物的 hmARCred HOMO 和 LUMO 的理解揭示了这些氧化还原轨道之间潜在的π键相互作用,底物 LUMO 沿反应坐标的双电子占据激活 O-N 键进行切割并促进氧原子转移到 Mo 位点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号