当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organozinc Reagents: Highly Efficient Scalable Continuous Conversion in Various Concentrations and Reaction Types
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-01 , DOI: 10.1021/acs.oprd.4c00292 Lars Gössl, Kai Dahms, Gabriele Menges-Flanagan, Michael Maskos
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-01 , DOI: 10.1021/acs.oprd.4c00292 Lars Gössl, Kai Dahms, Gabriele Menges-Flanagan, Michael Maskos
|
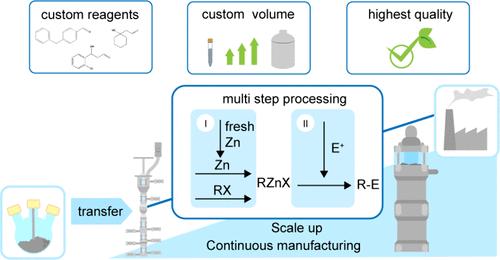
Organometallic reagents play a crucial role in today’s synthetic chemistry. They are used in the production of active pharmaceutical ingredients (APIs), fragrances, and agrochemicals, among other things, as they are instrumental and invaluable to form new carbon–carbon bonds. In addition to the widely used organolithium and organomagnesium compounds, better known as Grignard reagents, organozinc compounds are predestined coupling partners in C–C bond formation. Even though organozinc compounds are among the oldest organometallic compounds, they have long been superseded by the more reactive Grignard reagents (RMgX) and lithium organyls (RLi). The low reactivity of organozinc compounds in combination with a high sensitivity to oxygen and moisture lead to difficult handling and problematic storage. Their usefulness for C–C bond formation was therefore underestimated for a long time but has experienced a renaissance in recent decades. In a previous publication, the scalable continuous synthesis of organozinc compounds in different concentrations and solvents was demonstrated. The organozinc compounds were produced in both laboratory and pilot scale with good to very good yields and the formation of highly concentrated organozinc compounds was also confirmed. To build on this work, the continuous conversion of organozinc compounds is described below. Two different reaction types were investigated: the noncatalyzed Saytzeff reaction and the palladium-catalyzed Negishi cross-coupling reaction. The former was carried out in both a two-step and a one-pot approach. The reactive allylzinc bromide was chosen as the organometallic reagent, which was reacted with various aldehydes and ketones to yield secondary or tertiary homoallyl alcohols. In the Saytzeff reaction, residence times of 2.0 min were sufficient to achieve complete conversion of the carbonyl compound and isolated yields of 66–98%. The conversion of the carbonyl compound was monitored using an online process IR spectrometer with flow cell. In the case of the Negishi coupling, a fixed-bed reactor filled with Pd catalyst was used. The syntheses investigated were focused on the reaction of benzylzinc bromide with various functionalized organic halides. The Negishi coupling provided complete to near complete conversion of the electrophilic substrate with isolated yields of 72–92% at residence times of 23–32 s. Both the Saytzeff and Negishi reactions were extended to include the conversion of highly concentrated 2.0 M organozinc compounds. The former delivered yields of 83% and 92%, the latter 72% and 79%. The Saytzeff conversion was additionally transferred to pilot scale to demonstrate the ease of scalability. The synthesis of two selected compounds was successfully transferred to pilot scale, where a liquid throughput of 13 L/h was achieved. The main objective of this work was to establish various catalyzed and noncatalyzed conversions of organozinc reagents, particularly at high organozinc reagent concentrations to enable fast and safe process intensification, optimization and scalability to industrially relevant throughputs.
中文翻译:

有机锌试剂:各种浓度和反应类型的高效可扩展连续转化
有机金属试剂在当今的合成化学中起着至关重要的作用。它们用于生产活性药物成分 (API)、香料和农用化学品等,因为它们对于形成新的碳-碳键具有重要价值。除了广泛使用的有机锂和有机镁化合物(更广为人知的是格氏试剂)之外,有机锌化合物是 C-C 键形成的预定偶联伙伴。尽管有机锌化合物是最古老的有机金属化合物之一,但它们长期以来已被反应性更强的格氏试剂 (RMgX) 和锂有机基 (RLi) 所取代。有机锌化合物的低反应性与对氧气和水分的高度敏感性相结合,导致难以处理和储存问题。因此,它们在 C-C 键形成方面的有用性在很长一段时间内被低估了,但近几十年来经历了复兴。在之前的出版物中,展示了不同浓度和溶剂的有机锌化合物的可扩展连续合成。有机锌化合物在实验室和中试规模中均以良好到非常好的收率生产,并且还证实了高浓度有机锌化合物的形成。为了在这项工作的基础上,下面描述了有机锌化合物的连续转化。研究了两种不同的反应类型:非催化的 Saytzeff 反应和钯催化的 Negishi 交叉偶联反应。前者以两步法和一罐法进行。选择反应性烯丙基溴化锌作为有机金属试剂,与各种醛和酮反应,生成仲或叔均烯丙醇。 在 Saytzeff 反应中,2.0 min 的停留时间足以实现羰基化合物的完全转化,分离产率为 66–98%。使用带有流通池的在线在线红外光谱仪监测羰基化合物的转化率。在 Negishi 偶联的情况下,使用了填充有 Pd 催化剂的固定床反应器。研究的合成集中在溴化苄基锌与各种官能化有机卤化物的反应上。Negishi 偶联提供了亲电底物的完全到接近完全的转化,在 23-32 秒的停留时间内,分离产率为 72-92%。Saytzeff 和 Negishi 反应均扩展为包括高浓度 2.0 M 有机锌化合物的转化。前者的收益率分别为 83% 和 92%,后者分别为 72% 和 79%。Saytzeff 转换还被转移到试点规模,以展示可扩展性的易用性。两种选定化合物的合成成功转移到中试规模,液体通量达到 13 L/h。这项工作的主要目标是建立有机锌试剂的各种催化和非催化转化,特别是在高有机锌试剂浓度下,以实现快速和安全的工艺强化、优化和可扩展性,以实现工业相关通量。
更新日期:2024-10-01
中文翻译:

有机锌试剂:各种浓度和反应类型的高效可扩展连续转化
有机金属试剂在当今的合成化学中起着至关重要的作用。它们用于生产活性药物成分 (API)、香料和农用化学品等,因为它们对于形成新的碳-碳键具有重要价值。除了广泛使用的有机锂和有机镁化合物(更广为人知的是格氏试剂)之外,有机锌化合物是 C-C 键形成的预定偶联伙伴。尽管有机锌化合物是最古老的有机金属化合物之一,但它们长期以来已被反应性更强的格氏试剂 (RMgX) 和锂有机基 (RLi) 所取代。有机锌化合物的低反应性与对氧气和水分的高度敏感性相结合,导致难以处理和储存问题。因此,它们在 C-C 键形成方面的有用性在很长一段时间内被低估了,但近几十年来经历了复兴。在之前的出版物中,展示了不同浓度和溶剂的有机锌化合物的可扩展连续合成。有机锌化合物在实验室和中试规模中均以良好到非常好的收率生产,并且还证实了高浓度有机锌化合物的形成。为了在这项工作的基础上,下面描述了有机锌化合物的连续转化。研究了两种不同的反应类型:非催化的 Saytzeff 反应和钯催化的 Negishi 交叉偶联反应。前者以两步法和一罐法进行。选择反应性烯丙基溴化锌作为有机金属试剂,与各种醛和酮反应,生成仲或叔均烯丙醇。 在 Saytzeff 反应中,2.0 min 的停留时间足以实现羰基化合物的完全转化,分离产率为 66–98%。使用带有流通池的在线在线红外光谱仪监测羰基化合物的转化率。在 Negishi 偶联的情况下,使用了填充有 Pd 催化剂的固定床反应器。研究的合成集中在溴化苄基锌与各种官能化有机卤化物的反应上。Negishi 偶联提供了亲电底物的完全到接近完全的转化,在 23-32 秒的停留时间内,分离产率为 72-92%。Saytzeff 和 Negishi 反应均扩展为包括高浓度 2.0 M 有机锌化合物的转化。前者的收益率分别为 83% 和 92%,后者分别为 72% 和 79%。Saytzeff 转换还被转移到试点规模,以展示可扩展性的易用性。两种选定化合物的合成成功转移到中试规模,液体通量达到 13 L/h。这项工作的主要目标是建立有机锌试剂的各种催化和非催化转化,特别是在高有机锌试剂浓度下,以实现快速和安全的工艺强化、优化和可扩展性,以实现工业相关通量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号