当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal Effect on the Proton Conduction of Three Isostructural Metal–Organic Frameworks and Pseudo-Capacitance Behavior of the Cobalt Analogue
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-29 , DOI: 10.1021/acs.inorgchem.4c02958 Meng-Qian Yu, Cai-Yi Yang, Lian-Jun Dong, Yong Yan, Yu-Jie Feng, Zhongyan Chen, Hong-Ping Xiao, Hai-Ying Wang, Jing-Yuan Ge
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-29 , DOI: 10.1021/acs.inorgchem.4c02958 Meng-Qian Yu, Cai-Yi Yang, Lian-Jun Dong, Yong Yan, Yu-Jie Feng, Zhongyan Chen, Hong-Ping Xiao, Hai-Ying Wang, Jing-Yuan Ge
|
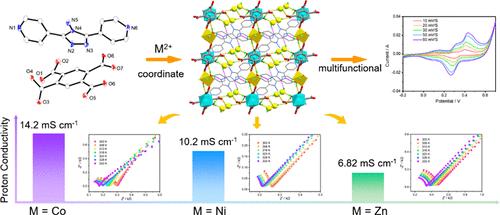
Three isostructural transition metal–organic frameworks, [M(bta)0.5(bpt)(H2O)2]·2H2O (M = Co (1), Ni (2), Zn (3), H4bta = 1,2,4,5-benzenetetracarboxylic acid, bpt = 4-amino-3,5-bis(4-pyridyl)-1,2,4-triazole), were successfully constructed using different metal cations. These frameworks exhibit a three-dimensional network structure with multiple coordinated and lattice water molecules within the framework, contributing to high stability and a rich hydrogen-bond network. Proton conduction studies revealed that, at 333 K and 98% relative humidity, the proton conductivities (σ) of MOFs 1–3 reached 1.42 × 10–2, 1.02 × 10–2, and 6.82 × 10–3 S cm–1, respectively. Compared to the proton conductivity of the initial ligands, the σ values of the complexes increased by 2 orders of magnitude, with the activation energies decreasing from 0.36 to 0.18 eV for 1, 0.09 eV for 2, and 0.12 eV for 3. An in-depth analysis of the correlation between different metal centers and proton conduction performance indicated that the varying coordination abilities of the metal cations and the water absorption capacities of the frameworks might account for the differences in conductivity. Additionally, the potential of 1 as a supercapacitor electrode material was assessed. 1 exhibited a specific capacitance of 61.13 F g–1 at a current density of 0.5 A g–1, with a capacitance retention of 82.4% after 5000 cycles, making it a promising candidate for energy storage applications.
中文翻译:

金属对三种等结构金属-有机框架质子传导的影响和钴类似物的赝电容行为
使用不同的金属阳离子成功构建了三种等结构过渡金属有机框架,[M(bta)0.5(bpt)(H2O)2]·2H2O (M = Co (1), Ni (2), Zn (3), H4bta = 1,2,4,5-苯四羧酸,bpt = 4-氨基-3,5-双(4-吡啶基)-1,2,4-三唑)。这些框架表现出三维网络结构,框架内具有多个配位和晶格水分子,有助于高稳定性和丰富的氢键网络。质子传导研究表明,在 333 K 和 98% 相对湿度下,MOF 1-3 的质子电导率 (σ) 分别达到 1.42 × 10-2、1.02 × 10-2 和 6.82 × 10-3 S cm–1。与初始配体的质子电导率相比,复合物的σ值增加了 2 个数量级,活化能从 1 的 0.36 降至 0.18 eV,2 的活化能从 0.09 eV 下降到 3 的 0.12 eV。对不同金属中心与质子传导性能之间相关性的深入分析表明,金属阳离子的不同配位能力和框架的吸水能力可能是电导率差异的原因。此外,还评估了 1 作为超级电容器电极材料的电位。1 在 0.5 A g–1 的电流密度下表现出 61.13 F g–1 的比电容,在 5000 次循环后电容保持率为 82.4%,使其成为储能应用的有前途的候选者。
更新日期:2024-09-29
中文翻译:

金属对三种等结构金属-有机框架质子传导的影响和钴类似物的赝电容行为
使用不同的金属阳离子成功构建了三种等结构过渡金属有机框架,[M(bta)0.5(bpt)(H2O)2]·2H2O (M = Co (1), Ni (2), Zn (3), H4bta = 1,2,4,5-苯四羧酸,bpt = 4-氨基-3,5-双(4-吡啶基)-1,2,4-三唑)。这些框架表现出三维网络结构,框架内具有多个配位和晶格水分子,有助于高稳定性和丰富的氢键网络。质子传导研究表明,在 333 K 和 98% 相对湿度下,MOF 1-3 的质子电导率 (σ) 分别达到 1.42 × 10-2、1.02 × 10-2 和 6.82 × 10-3 S cm–1。与初始配体的质子电导率相比,复合物的σ值增加了 2 个数量级,活化能从 1 的 0.36 降至 0.18 eV,2 的活化能从 0.09 eV 下降到 3 的 0.12 eV。对不同金属中心与质子传导性能之间相关性的深入分析表明,金属阳离子的不同配位能力和框架的吸水能力可能是电导率差异的原因。此外,还评估了 1 作为超级电容器电极材料的电位。1 在 0.5 A g–1 的电流密度下表现出 61.13 F g–1 的比电容,在 5000 次循环后电容保持率为 82.4%,使其成为储能应用的有前途的候选者。






























 京公网安备 11010802027423号
京公网安备 11010802027423号