当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Linear Free Energy Relationships Associated with Hydride Transfer From [(6,6′-R2-bpy)Re(CO)3H]: A Cautionary Tale in Identifying Hydrogen Bonding Effects in the Secondary Coordination Sphere
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-30 , DOI: 10.1021/acs.inorgchem.4c03365 Matthew R. Elsby, Abhishek Kumar, Lee M. Daniels, Mehmed Z. Ertem, Nilay Hazari, Brandon Q. Mercado, Alexandra H. Paulus
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-30 , DOI: 10.1021/acs.inorgchem.4c03365 Matthew R. Elsby, Abhishek Kumar, Lee M. Daniels, Mehmed Z. Ertem, Nilay Hazari, Brandon Q. Mercado, Alexandra H. Paulus
|
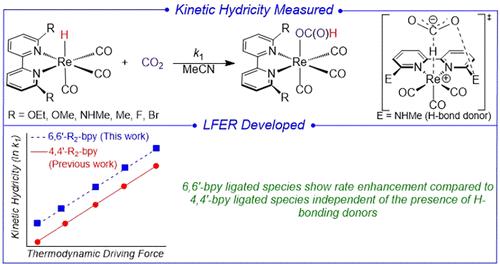
Six rhenium hydride complexes, [(6,6′-R2-bpy)Re(CO)3H] (bpy = 2,2′-bipyridine, R = OEt, OMe, NHMe, Me, F, Br), were synthesized. These complexes insert CO2 to form rhenium formate complexes of the type [(6,6′-R2-bpy)Re(CO)3{OC(O)H}]. All the rhenium formate species were characterized using X-ray crystallography, which revealed that the bpy ligand is not coplanar with the metal coordination plane containing the two nitrogen donors of the bpy ligand but tilted. A solid-state structure of [(6,6′-Me2-bpy)Re(CO)3H] determined using MicroED also featured a tilted bpy ligand. The kinetics of CO2 insertion into complexes of the type [(6,6′-R2-bpy)Re(CO)3H] were measured experimentally and the thermodynamic hydricities of [(6,6′-R2-bpy)Re(CO)3H] species were determined using theoretical calculations. A Brønsted plot constructed using the experimentally determined rate constants for CO2 insertion and the calculated thermodynamic hydricities for [(6,6′-R2-bpy)Re(CO)3H] revealed a linear free energy relationship (LFER) between thermodynamic and kinetic hydricity. This LFER is different to the previously determined relationship for CO2 insertion into complexes of the type [(4,4′-R2-bpy)Re(CO)3H]. At a given thermodynamic hydricity, CO2 insertion is faster for complexes containing a 6,6′-substituted bpy ligand. This is likely in part due to the tilting observed for systems with 6,6′-substituted bpy ligands. Notably, the 6,6′-(NHMe)2-bpy ligand could in principle stabilize the transition state for CO2 insertion via hydrogen bonding. This work shows that if only the rate of CO2 insertion into [(6,6′-(NHMe)2-bpy)Re(CO)3H] is compared to [(4,4′-R2-bpy)Re(CO)3H] systems, the increase in rate could be easily attributed to hydrogen bonding, but in fact all 6,6′-substituted systems lead to faster than expected rates.
中文翻译:

与从 [(6,6′-R2-bpy)Re(CO)3H] 的氢化物转移相关的线性自由能关系:识别次级配位球中氢键效应的警示故事
合成了六种氢化铼配合物 [(6,6′-R2-bpy)Re(CO)3H] (bpy = 2,2′-联吡啶,R = OEt, OMe, NHMe, Me, F, Br)。这些配合物插入 CO2 形成 [(6,6′-R 2-bpy)Re(CO)3{OC(O)H}] 型甲酸铼配合物。使用 X 射线晶体学对所有甲酸铼物种进行了表征,结果表明 bpy 配体与包含 bpy 配体的两个氮供体的金属配位面不共面,而是倾斜的。使用 MicroED 测定的 [(6,6′-Me2-bpy)Re(CO)3H] 的固态结构也具有倾斜的 bpy 配体。通过实验测量了 CO2 插入 [(6,6′-R 2-bpy)Re(CO)3H] 型复合物中的动力学,并使用理论计算确定了 [(6,6′-R2-bpy)Re(CO)3H] 物种的热力学水合度。使用实验确定的 CO2 插入速率常数和计算的 [(6,6′-R 2-bpy)Re(CO)3H] 的热力学水力度构建的 Brønsted 图揭示了热力学和动力学水力之间的线性自由能关系 (LFER)。该 LFER 与先前确定的 CO2 插入 [(4,4′-R 2-bpy)Re(CO)3H] 型复合物的关系不同。在给定的热力学水力下,对于包含 6,6′-取代的 bpy 配体的复合物,CO2 插入更快。这可能部分是由于在具有 6,6′ 取代的 bpy 配体的系统中观察到的倾斜。 值得注意的是,6,6′-(NHMe)2-bpy 配体原则上可以通过氢键稳定 CO2 插入的过渡态。这项工作表明,如果仅将 CO2 插入 [(6,6′-(NHMe)2-bpy)Re(CO)3H] 的速率与 [(4,4′-R2-bpy)Re(CO)3H] 系统进行比较,速率的增加可以很容易地归因于氢键,但实际上所有 6,6′-取代的系统都会导致比预期更快的速率。
更新日期:2024-09-30
中文翻译:

与从 [(6,6′-R2-bpy)Re(CO)3H] 的氢化物转移相关的线性自由能关系:识别次级配位球中氢键效应的警示故事
合成了六种氢化铼配合物 [(6,6′-R2-bpy)Re(CO)3H] (bpy = 2,2′-联吡啶,R = OEt, OMe, NHMe, Me, F, Br)。这些配合物插入 CO2 形成 [(6,6′-R 2-bpy)Re(CO)3{OC(O)H}] 型甲酸铼配合物。使用 X 射线晶体学对所有甲酸铼物种进行了表征,结果表明 bpy 配体与包含 bpy 配体的两个氮供体的金属配位面不共面,而是倾斜的。使用 MicroED 测定的 [(6,6′-Me2-bpy)Re(CO)3H] 的固态结构也具有倾斜的 bpy 配体。通过实验测量了 CO2 插入 [(6,6′-R 2-bpy)Re(CO)3H] 型复合物中的动力学,并使用理论计算确定了 [(6,6′-R2-bpy)Re(CO)3H] 物种的热力学水合度。使用实验确定的 CO2 插入速率常数和计算的 [(6,6′-R 2-bpy)Re(CO)3H] 的热力学水力度构建的 Brønsted 图揭示了热力学和动力学水力之间的线性自由能关系 (LFER)。该 LFER 与先前确定的 CO2 插入 [(4,4′-R 2-bpy)Re(CO)3H] 型复合物的关系不同。在给定的热力学水力下,对于包含 6,6′-取代的 bpy 配体的复合物,CO2 插入更快。这可能部分是由于在具有 6,6′ 取代的 bpy 配体的系统中观察到的倾斜。 值得注意的是,6,6′-(NHMe)2-bpy 配体原则上可以通过氢键稳定 CO2 插入的过渡态。这项工作表明,如果仅将 CO2 插入 [(6,6′-(NHMe)2-bpy)Re(CO)3H] 的速率与 [(4,4′-R2-bpy)Re(CO)3H] 系统进行比较,速率的增加可以很容易地归因于氢键,但实际上所有 6,6′-取代的系统都会导致比预期更快的速率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号