当前位置:
X-MOL 学术
›
Pest Manag. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel heterocyclic amide derivatives containing a diphenylmethyl moiety: systematic optimizations, synthesis, antifungal activity and action mechanism
Pest Management Science ( IF 3.8 ) Pub Date : 2024-09-30 , DOI: 10.1002/ps.8448 Feng Peng, Jianqi Chai, Yue Xie, Lang Tai, Min Chen, Chunlong Yang
Pest Management Science ( IF 3.8 ) Pub Date : 2024-09-30 , DOI: 10.1002/ps.8448 Feng Peng, Jianqi Chai, Yue Xie, Lang Tai, Min Chen, Chunlong Yang
|
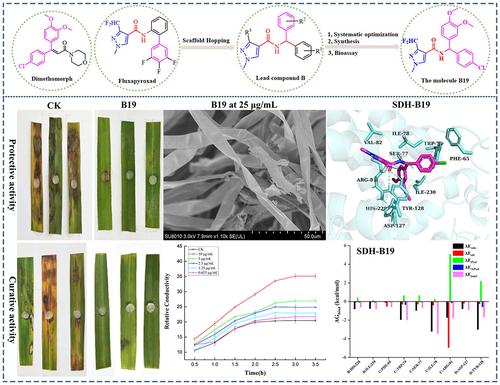
The development of fungicides with low cross resistance, high efficacy and low resistance plays a central role in protecting crops, reducing yield losses, improving quality and maintaining global food security. Based on this important role, after a systematic optimization strategy, novel heterocyclic amide derivatives bearing diphenylmethyl fragment were screened, synthesized and verified with the spectrographic and x-ray diffraction analysis.
中文翻译:

含有二苯基甲基基团的新型杂环酰胺衍生物:系统优化、合成、抗真菌活性和作用机制
开发低交叉抗性、高效和低抗性的杀菌剂在保护作物、减少产量损失、提高质量和维护全球粮食安全方面发挥着核心作用。基于这一重要作用,经过系统优化策略,筛选、合成了带有二苯甲基片段的新型杂环酰胺衍生物,并通过光谱和 X 射线衍射分析进行了验证。
更新日期:2024-09-30
中文翻译:

含有二苯基甲基基团的新型杂环酰胺衍生物:系统优化、合成、抗真菌活性和作用机制
开发低交叉抗性、高效和低抗性的杀菌剂在保护作物、减少产量损失、提高质量和维护全球粮食安全方面发挥着核心作用。基于这一重要作用,经过系统优化策略,筛选、合成了带有二苯甲基片段的新型杂环酰胺衍生物,并通过光谱和 X 射线衍射分析进行了验证。

































 京公网安备 11010802027423号
京公网安备 11010802027423号