当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Enantiopure Fluoropiperidines via Biocatalytic Desymmetrization and Flow Photochemical Decarboxylative Fluorination
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-30 , DOI: 10.1021/acs.oprd.4c00139 Caroline A. Blakemore, John M. Humphrey, Eddie Yang, Jeffrey T. Kohrt, Peter Daniel Morse, Roger M. Howard, Hatice G. Yayla, Thomas Knauber, Longfei Xie, Teresa Makowski, Jeffrey W. Raggon, Rebecca B. Watson, Christopher W. am Ende, Tim Ryder, Ormacinda White, Martin R. M. Koos, Rajesh Kumar, Feng Shi, Jie Li, Huan Wang, Like Chen, Julai Wang
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-30 , DOI: 10.1021/acs.oprd.4c00139 Caroline A. Blakemore, John M. Humphrey, Eddie Yang, Jeffrey T. Kohrt, Peter Daniel Morse, Roger M. Howard, Hatice G. Yayla, Thomas Knauber, Longfei Xie, Teresa Makowski, Jeffrey W. Raggon, Rebecca B. Watson, Christopher W. am Ende, Tim Ryder, Ormacinda White, Martin R. M. Koos, Rajesh Kumar, Feng Shi, Jie Li, Huan Wang, Like Chen, Julai Wang
|
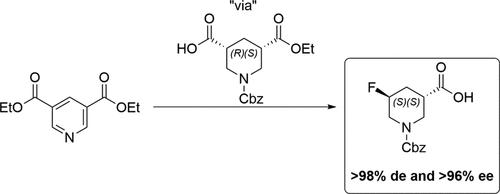
Low-molecular weight chiral amines are valuable components in medicinal chemistry as they serve as core templates, linking units, and substituent appendages. The piperidine scaffold is particularly useful among privileged small amines, with substituted variants having a great number of potential regio- and diastereoisomers, which allow for high stereochemical definition to enable a variety of productive protein interactions. Herein, we describe the successful enablement, scale-up, and delivery of >400 g of a single isomer, (3S,5S)-1-((benzyloxy)carbonyl)-5-fluoropiperidine-3-carboxylic acid (>98% de and >96% ee), via 450 g-scale biocatalytic desymmetrization and 335 g-scale flow photochemical decarboxylative fluorination.
中文翻译:

通过生物催化去对称和流动光化学脱羧氟化合成对映体纯氟哌啶
低分子量手性胺是药物化学中有价值的组分,因为它们用作核心模板、连接单元和取代基附属物。哌啶支架在特权小胺中特别有用,取代变体具有大量潜在的区域和非对映异构体,可实现高立体化学清晰度,以实现各种高效的蛋白质相互作用。在本文中,我们描述了通过 450 g 级生物催化去对称化和 335 g 级光化学脱羧氟化成功实现、放大和交付 >400 g 单一异构体,(3S,5 S)-1-((苄氧基)羰基)-5-氟哌啶-3-羧酸(>98% de 和 >96% ee)。
更新日期:2024-09-30
中文翻译:

通过生物催化去对称和流动光化学脱羧氟化合成对映体纯氟哌啶
低分子量手性胺是药物化学中有价值的组分,因为它们用作核心模板、连接单元和取代基附属物。哌啶支架在特权小胺中特别有用,取代变体具有大量潜在的区域和非对映异构体,可实现高立体化学清晰度,以实现各种高效的蛋白质相互作用。在本文中,我们描述了通过 450 g 级生物催化去对称化和 335 g 级光化学脱羧氟化成功实现、放大和交付 >400 g 单一异构体,(3S,5 S)-1-((苄氧基)羰基)-5-氟哌啶-3-羧酸(>98% de 和 >96% ee)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号