当前位置:
X-MOL 学术
›
J. Magnes. Alloys
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ formation of an intimate solid-solid interface by reaction between MgH2 and Ti to stabilize metal hydride anode with high active material content
Journal of Magnesium and Alloys ( IF 15.8 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.jma.2024.08.006 Yixin Chen, Atsushi Inoishi, Shigeto Okada, Hikari Sakaebe, Ken Albrecht
Journal of Magnesium and Alloys ( IF 15.8 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.jma.2024.08.006 Yixin Chen, Atsushi Inoishi, Shigeto Okada, Hikari Sakaebe, Ken Albrecht
|
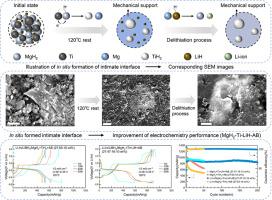
MgH2 and TiH2 have been extensively studied as potential anode materials due to their high theoretical specific capacities of 2036 and 1024 mAh/g, respectively. However, the large volume changes that these compounds undergo during cycling affects their performance and limits practical applications. The present work demonstrates a novel approach to limiting the volume changes of active materials. This effect is based on mechanical support from an intimate interface generated in situ via the reaction between MgH2 and Ti within the electrode prior to lithiation to form Mg and TiH2. The resulting Mg can be transformed back to MgH2 by reaction with LiH during delithiation. In addition, the TiH2 improves the reaction kinetics of MgH2 and enhances electrochemical performance. The intimate interface produced in this manner is found to improve the electrochemical properties of a MgH2-Ti-LiH electrode. An exceptional reversible capacity of 800 mAh/g is observed even after 200 cycles with a high current density of 1 mA/cm2 and a high proportion of active material (90 wt.%) at an operation temperature of 120 °C. This study therefore showcases a new means of improving the performance of electrodes by limiting the volume changes of active materials.
中文翻译:

通过 MgH2 和 Ti 之间的反应原位形成紧密的固-固界面,以稳定具有高活性材料含量的金属氢化物阳极
MgH2 和 TiH2 作为潜在的负极材料已被广泛研究,因为它们分别具有 2036 和 1024 mAh/g 的高理论比容量。然而,这些化合物在循环过程中经历的大量体积变化会影响其性能并限制实际应用。这项工作展示了一种限制活性材料体积变化的新方法。这种效应基于在锂化形成 Mg 和 TiH2 之前,电极内 MgH2 和 Ti 之间的反应原位产生的紧密界面的机械支撑。在脱锂过程中,通过与 LiH 反应,所得的 Mg 可以转化回 MgH2。此外,TiH2 改善了 MgH2 的反应动力学并增强了电化学性能。以这种方式产生的亲密界面被发现可以改善 MgH 2-Ti-LiH 电极的电化学性能。在 120 °C 的工作温度下,即使在 1 mA/cm2 的高电流密度和高比例活性材料 (90 wt.%) 的 200 次循环后,也能观察到 800 mAh/g 的出色可逆容量。 因此,本研究展示了一种通过限制活性材料的体积变化来提高电极性能的新方法。
更新日期:2024-09-05
中文翻译:

通过 MgH2 和 Ti 之间的反应原位形成紧密的固-固界面,以稳定具有高活性材料含量的金属氢化物阳极
MgH2 和 TiH2 作为潜在的负极材料已被广泛研究,因为它们分别具有 2036 和 1024 mAh/g 的高理论比容量。然而,这些化合物在循环过程中经历的大量体积变化会影响其性能并限制实际应用。这项工作展示了一种限制活性材料体积变化的新方法。这种效应基于在锂化形成 Mg 和 TiH2 之前,电极内 MgH2 和 Ti 之间的反应原位产生的紧密界面的机械支撑。在脱锂过程中,通过与 LiH 反应,所得的 Mg 可以转化回 MgH2。此外,TiH2 改善了 MgH2 的反应动力学并增强了电化学性能。以这种方式产生的亲密界面被发现可以改善 MgH 2-Ti-LiH 电极的电化学性能。在 120 °C 的工作温度下,即使在 1 mA/cm2 的高电流密度和高比例活性材料 (90 wt.%) 的 200 次循环后,也能观察到 800 mAh/g 的出色可逆容量。 因此,本研究展示了一种通过限制活性材料的体积变化来提高电极性能的新方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号