当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydride and halide abstraction reactions behind the enhanced basicity of Be and Mg clusters with nitrogen bases
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-28 , DOI: 10.1002/jcc.27509 Manuel Yáñez, Otilia Mó, M. Merced Montero-Campillo, Ibon Alkorta, José Elguero
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-28 , DOI: 10.1002/jcc.27509 Manuel Yáñez, Otilia Mó, M. Merced Montero-Campillo, Ibon Alkorta, José Elguero
|
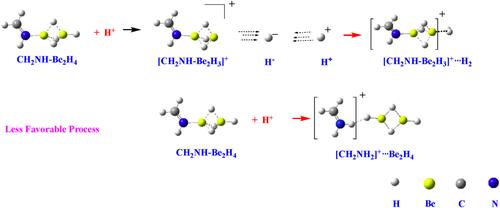
In this study, we investigate the protonation effects on the structure, relative stability and basicity of complexes formed by the interaction of monomers and dimers of BeX2 and MgX2 (X = H, F) with NH3, CH2NH, HCN, and NC5H5 bases. Calculations were performed using the M06-2X/aug-cc-pVTZ formalism, along with QTAIM, ELF and NCI methods for electron density analysis and MBIE and LMO-EDA energy decomposition analyses for interaction enthalpies. The protonation of the MH2– and M2H4–Base complexes occurs at the negatively charged hydrogen atoms of the MH2 and M2H4 moieties through typical hydride abstraction reactions, while protonation at the N atom of the base is systematically less exothermic. The preference for the hydride transfer mechanism is directly associated with the significant exothermicity of H2 formation through the interaction between H− and H+, and the high hydride donor ability of these complexes. The basicity of both, MH2 and M2H4 compounds increases enormously upon association with the corresponding bases, with the increase exceeding 40 orders of magnitude in terms of ionization constants. Due to the smaller exothermicity of HF formation, the basicity of fluorides is lower than that of hydrides. In Be complexes, the protonation at the N atom of the base dominates over the fluoride abstraction mechanism. However, for the Mg complexes the fluoride abstraction mechanism is energetically the most favorable process, reflecting the greater facility of Mg complexes to lose F−.
中文翻译:

含氮碱的 Be 和 Mg 团簇碱度增强背后的氢化物和卤化物萃取反应
在这项研究中,我们研究了 BeX2 和 MgX2 (X = H, F) 的单体和二聚体与 NH3、CH2NH、HCN 和 NC5H5 碱基相互作用形成的复合物的质子化影响。使用 M06-2X/aug-cc-pVTZ 形式进行计算,并使用 QTAIM、ELF 和 NCI 方法进行电子密度分析,使用 MBIE 和 LMO-EDA 能量分解分析进行相互作用焓。MH2 和 M2H4 碱基复合物的质子化通过典型的氢化物萃取反应发生在 MH2 和 M2H4 部分的带负电荷的氢原子上,而碱基 N 原子的质子化在系统上放热性较低。对氢化物转移机制的偏好与通过 H− 和 H+ 之间的相互作用形成 H2 的显着放热性以及这些复合物的高氢化物供体能力直接相关。MH2 和 M2H4 化合物的碱度在与相应的碱基结合时显著增加,电离常数增加超过 40 个数量级。由于 HF 形成的放热性较小,氟化物的碱度低于氢化物。在 Be 配合物中,碱基 N 原子处的质子化主导了氟化物提取机制。然而,对于 Mg 配合物,氟化物提取机制在能量上是最有利的过程,这反映了 Mg 配合物更易失去 F−。
更新日期:2024-09-28
中文翻译:

含氮碱的 Be 和 Mg 团簇碱度增强背后的氢化物和卤化物萃取反应
在这项研究中,我们研究了 BeX2 和 MgX2 (X = H, F) 的单体和二聚体与 NH3、CH2NH、HCN 和 NC5H5 碱基相互作用形成的复合物的质子化影响。使用 M06-2X/aug-cc-pVTZ 形式进行计算,并使用 QTAIM、ELF 和 NCI 方法进行电子密度分析,使用 MBIE 和 LMO-EDA 能量分解分析进行相互作用焓。MH2 和 M2H4 碱基复合物的质子化通过典型的氢化物萃取反应发生在 MH2 和 M2H4 部分的带负电荷的氢原子上,而碱基 N 原子的质子化在系统上放热性较低。对氢化物转移机制的偏好与通过 H− 和 H+ 之间的相互作用形成 H2 的显着放热性以及这些复合物的高氢化物供体能力直接相关。MH2 和 M2H4 化合物的碱度在与相应的碱基结合时显著增加,电离常数增加超过 40 个数量级。由于 HF 形成的放热性较小,氟化物的碱度低于氢化物。在 Be 配合物中,碱基 N 原子处的质子化主导了氟化物提取机制。然而,对于 Mg 配合物,氟化物提取机制在能量上是最有利的过程,这反映了 Mg 配合物更易失去 F−。































 京公网安备 11010802027423号
京公网安备 11010802027423号