当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization and Solid-State UV–Vis Investigations of Photoelectrocatalytically Active La5Cl7[TeO3]4, a Mixed Anion Compound with Alternating 2D Layers of Oxygen and Chlorine
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-27 , DOI: 10.1021/acs.inorgchem.4c02392 Johnny A. Sannes, Athanasios Chatzitakis, Emil H. Fro̷en, Niels Ho̷jmark Andersen, Ola Nilsen, Martin Valldor
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-09-27 , DOI: 10.1021/acs.inorgchem.4c02392 Johnny A. Sannes, Athanasios Chatzitakis, Emil H. Fro̷en, Niels Ho̷jmark Andersen, Ola Nilsen, Martin Valldor
|
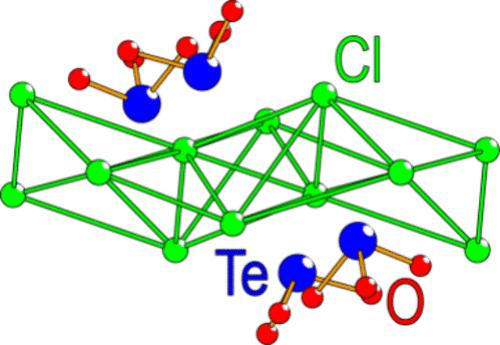
An oxide chloride, La5Cl7[TeO3]4, was synthesized using the conventional high-temperature solid-state synthesis technique in an inert atmosphere. This compound possesses a novel crystal structure that can be described with the triclinic space group P1̅ (No. 2) and unit cell parameters: a = 7.2634(3) Å, b = 8.1241(3) Å, c = 9.1993(3) Å, α = 79.373(1)°, β = 83.599(1)°, and γ = 82.511(1)°. The preference of Te(IV) to coordinate to oxygen and direct its lone pair toward the lower charged chlorine results in 2D layers of both oxygen and chlorine, alternating along the crystallographic b-direction. Homoleptic coordination, solely to oxygen, and heteroleptic coordination to oxygen and chlorine are observed for lanthanum, forming layers connected through edge-sharing polyhedra. In the crystal structure, two distinct tellurium positions are observed, with three close Te–O distances, emphasizing an active lone pair. The compound has been investigated by solid-state UV–vis measurements, and a band gap of 3.44 eV has been determined by DFT calculations. Detailed photoelectrochemical measurements clearly indicate that the title compound is photoelectrocatalytically active, showing an n-type behavior. Raman spectroscopy confirms that complex tellurite ions are present in the crystal structure; several observed bands can be assigned to Te–O stretching, reflecting the relatively low crystallographic symmetry of the title compound.
中文翻译:

光电催化活性 La5Cl7[TeO3]4 的表征和固态紫外-可见光研究,La5Cl7[TeO3]4 是一种具有交替 2D 氧和氯层的混合阴离子化合物
在惰性气氛中,使用常规的高温固相合成技术合成了一氧化氯 La5Cl7[TeO3]4。该化合物具有新颖的晶体结构,可以用三斜空间群 P1̅(编号 2)和晶胞参数来描述:a = 7.2634(3) Å、b = 8.1241(3) Å、c = 9.1993(3) Å、α = 79.373(1)°、β = 83.599(1)° 和 γ = 82.511(1)°。Te(IV) 倾向于配位氧并将其孤对电子指向电荷较低的氯,导致氧和氯的 2D 层沿晶体学 b 方向交替。观察到镧的同态配位(仅与氧)和与氧和氯的异质配位,形成通过共享边缘多面体连接的层。在晶体结构中,观察到两个不同的碲位置,具有三个紧密的 Te-O 距离,强调活跃的孤对电子。该化合物已通过固态紫外-可见光测量进行了研究,并通过 DFT 计算确定了 3.44 eV 的带隙。详细的光电化学测量清楚地表明,标题化合物具有光电催化活性,显示出 n 型行为。拉曼光谱证实晶体结构中存在复杂的亚碲酸盐离子;几个观察到的条带可以归类为 Te-O 拉伸,这反映了标题化合物相对较低的晶体对称性。
更新日期:2024-09-27
中文翻译:

光电催化活性 La5Cl7[TeO3]4 的表征和固态紫外-可见光研究,La5Cl7[TeO3]4 是一种具有交替 2D 氧和氯层的混合阴离子化合物
在惰性气氛中,使用常规的高温固相合成技术合成了一氧化氯 La5Cl7[TeO3]4。该化合物具有新颖的晶体结构,可以用三斜空间群 P1̅(编号 2)和晶胞参数来描述:a = 7.2634(3) Å、b = 8.1241(3) Å、c = 9.1993(3) Å、α = 79.373(1)°、β = 83.599(1)° 和 γ = 82.511(1)°。Te(IV) 倾向于配位氧并将其孤对电子指向电荷较低的氯,导致氧和氯的 2D 层沿晶体学 b 方向交替。观察到镧的同态配位(仅与氧)和与氧和氯的异质配位,形成通过共享边缘多面体连接的层。在晶体结构中,观察到两个不同的碲位置,具有三个紧密的 Te-O 距离,强调活跃的孤对电子。该化合物已通过固态紫外-可见光测量进行了研究,并通过 DFT 计算确定了 3.44 eV 的带隙。详细的光电化学测量清楚地表明,标题化合物具有光电催化活性,显示出 n 型行为。拉曼光谱证实晶体结构中存在复杂的亚碲酸盐离子;几个观察到的条带可以归类为 Te-O 拉伸,这反映了标题化合物相对较低的晶体对称性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号