Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A reversible four-electron Sn metal aqueous battery
Joule ( IF 38.6 ) Pub Date : 2024-09-27 , DOI: 10.1016/j.joule.2024.09.002 Jianbo Wang, Sofia K. Catalina, Zhelong Jiang, Xin Xu, Qin Tracy Zhou, William C. Chueh, J. Tyler Mefford
Joule ( IF 38.6 ) Pub Date : 2024-09-27 , DOI: 10.1016/j.joule.2024.09.002 Jianbo Wang, Sofia K. Catalina, Zhelong Jiang, Xin Xu, Qin Tracy Zhou, William C. Chueh, J. Tyler Mefford
|
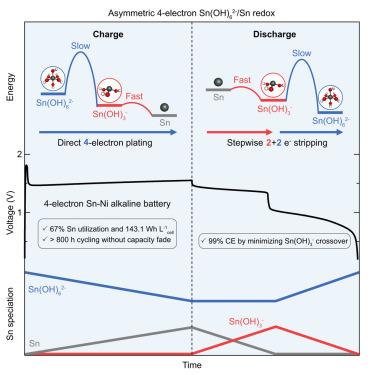
Sn is a promising metal anode for aqueous batteries, with up to four-electron redox available per atom (903 mAh g−1Sn). However, practically harnessing the four-electron Sn(OH)62−/Sn reversibility remains challenging due to limited mechanistic understanding. Here, we reveal a kinetically asymmetric redox pathway involving a successive four-electron plating and a stepwise 2 + 2 electron stripping through a Sn(OH)3− intermediate. The crossover of Sn(OH)3− induces a reversible self-discharge that reduces Coulombic efficiency but does not impact cyclability, demonstrated by four-electron Sn-Ni full cells that sustain >800 h of stable cycling. By tuning the ion selectivity of the separator to suppress Sn(OH)3− crossover while allowing OH− transport, we further demonstrate high Sn utilization (67%) and high energy density (143.1 Wh L−1cell). The results provide key understandings of the tradeoffs in engineering reversible multi-electron metal anodes and define a new benchmark for practical energy density that exceeds any Sn-based aqueous batteries to date.
中文翻译:

一种可逆四电子 Sn 金属水系电池
Sn 是一种很有前途的用于水系电池的金属负极,每个原子最多可产生四个电子的氧化还原 (903 mAh g-1Sn)。然而,由于对机理的理解有限,实际利用四电子 Sn(OH)62−/Sn 的可逆性仍然具有挑战性。在这里,我们揭示了一种动力学不对称的氧化还原途径,涉及连续的四电子电镀和通过 Sn(OH)3− 中间体的逐步 2 + 2 电子剥离。Sn(OH)3− 的交叉诱导可逆自放电,这降低了库仑效率,但不影响可循环性,维持 >800 h 稳定循环的四电子 Sn-Ni 全电池证明了这一点。通过调整隔膜的离子选择性以抑制 Sn(OH)3− 交叉,同时允许 OH− 传输,我们进一步证明了高 Sn 利用率 (67%) 和高能量密度(143.1 Wh L-1电池)。这些结果为工程可逆多电子金属负极的权衡提供了关键理解,并为实际能量密度定义了一个新的基准,该基准超过了迄今为止任何 Sn 基水系电池。
更新日期:2024-09-27
中文翻译:

一种可逆四电子 Sn 金属水系电池
Sn 是一种很有前途的用于水系电池的金属负极,每个原子最多可产生四个电子的氧化还原 (903 mAh g-1Sn)。然而,由于对机理的理解有限,实际利用四电子 Sn(OH)62−/Sn 的可逆性仍然具有挑战性。在这里,我们揭示了一种动力学不对称的氧化还原途径,涉及连续的四电子电镀和通过 Sn(OH)3− 中间体的逐步 2 + 2 电子剥离。Sn(OH)3− 的交叉诱导可逆自放电,这降低了库仑效率,但不影响可循环性,维持 >800 h 稳定循环的四电子 Sn-Ni 全电池证明了这一点。通过调整隔膜的离子选择性以抑制 Sn(OH)3− 交叉,同时允许 OH− 传输,我们进一步证明了高 Sn 利用率 (67%) 和高能量密度(143.1 Wh L-1电池)。这些结果为工程可逆多电子金属负极的权衡提供了关键理解,并为实际能量密度定义了一个新的基准,该基准超过了迄今为止任何 Sn 基水系电池。






























 京公网安备 11010802027423号
京公网安备 11010802027423号