当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective Electrosynthesis of 1H-1-Hydroxyquinol-4-ones–Synthetic Access to Versatile Natural Antibiotics
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-24 , DOI: 10.1021/acs.oprd.4c00337 Tobias Prenzel, Nils Schwarz, Jasmin Hammes, Franziska Krähe, Sarah Pschierer, Johannes Winter, María de Jesús Gálvez-Vázquez, Dieter Schollmeyer, Siegfried R. Waldvogel
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-09-24 , DOI: 10.1021/acs.oprd.4c00337 Tobias Prenzel, Nils Schwarz, Jasmin Hammes, Franziska Krähe, Sarah Pschierer, Johannes Winter, María de Jesús Gálvez-Vázquez, Dieter Schollmeyer, Siegfried R. Waldvogel
|
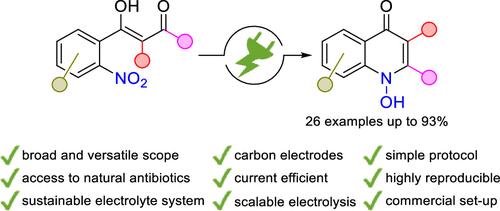
1H-1-Hydroxyquinolin-4-ones represent a broad class of biologically active heterocycles having an exocyclic N,O motif. Electrosynthesis offers direct, highly selective, and sustainable access to 1-hydroxyquinol-4-ones by nitro reduction. A versatile synthetic route starting from easily accessible 2-nitrobenzoic acids was established. The broad applicability of this protocol was demonstrated on 26 examples with up to 93% yield, highlighted by the naturally occurring antibiotics Aurachin C and HQNO. The practicability and technical relevance were underlined by multigram scale electrolysis.
中文翻译:

1H-1-羟基喹醇-4-酮合成的高选择性电合成,用于多种天然抗生素
1H-1-羟基喹啉-4-酮代表一大类具有外环 N,O 基序的生物活性杂环。电合成通过硝基还原提供了直接、高选择性和可持续的 1-羟基喹啉-4-酮。建立了一种从易于获得的 2-硝基苯甲酸开始的多功能合成路线。该方案的广泛适用性已在 26 个实例上得到证明,产量高达 93%,天然存在的抗生素 Aurachin C 和 HQNO 尤为突出。数克级电解强调了实用性和技术相关性。
更新日期:2024-09-24
中文翻译:

1H-1-羟基喹醇-4-酮合成的高选择性电合成,用于多种天然抗生素
1H-1-羟基喹啉-4-酮代表一大类具有外环 N,O 基序的生物活性杂环。电合成通过硝基还原提供了直接、高选择性和可持续的 1-羟基喹啉-4-酮。建立了一种从易于获得的 2-硝基苯甲酸开始的多功能合成路线。该方案的广泛适用性已在 26 个实例上得到证明,产量高达 93%,天然存在的抗生素 Aurachin C 和 HQNO 尤为突出。数克级电解强调了实用性和技术相关性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号