当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Building Catalytic Reactions One Electron at a Time
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-24 , DOI: 10.1021/acs.accounts.4c00515 Julian G. West
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-24 , DOI: 10.1021/acs.accounts.4c00515 Julian G. West
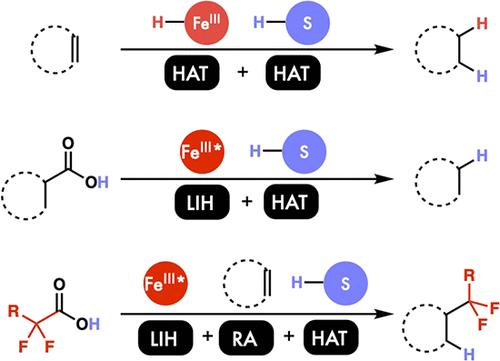
|
Classical education in organic chemistry and catalysis, not the least my own, has centered on two-electron transformations, from nucleophilic attack to oxidative addition. The focus on two-electron chemistry is well-founded, as this brand of chemistry has enabled incredible feats of synthesis, from the development of life-saving pharmaceuticals to the production of ubiquitous commodity chemicals. With that said, this approach is in many ways complementary to the approach of nature, where enzymes frequently make use of single-electron “radical” steps to achieve challenging reactions with exceptional selectivity, including light detection and C–H hydroxylation. While the power of radical elementary steps is undeniable, the fundamental understanding of─and ability to apply─these in catalysis remains underdeveloped, constraining the palette with which chemists can make new reactions.
中文翻译:

一次构建一个电子的催化反应
有机化学和催化的经典教育,尤其是我自己的教育,以双电子转变为中心,从亲核攻击到氧化加成。对双电子化学的关注是有根据的,因为这种化学品牌实现了令人难以置信的合成壮举,从开发拯救生命的药物到生产无处不在的商品化学品。话虽如此,这种方法在许多方面与自然界的方法相辅相成,其中酶经常利用单电子“自由基”步骤来实现具有出色选择性的具有挑战性的反应,包括光检测和 C-H 羟基化。虽然自由基基本步骤的力量是不可否认的,但对这些在催化中的基本理解和应用能力仍然不发达,这限制了化学家进行新反应的调色板。
更新日期:2024-09-24
中文翻译:

一次构建一个电子的催化反应
有机化学和催化的经典教育,尤其是我自己的教育,以双电子转变为中心,从亲核攻击到氧化加成。对双电子化学的关注是有根据的,因为这种化学品牌实现了令人难以置信的合成壮举,从开发拯救生命的药物到生产无处不在的商品化学品。话虽如此,这种方法在许多方面与自然界的方法相辅相成,其中酶经常利用单电子“自由基”步骤来实现具有出色选择性的具有挑战性的反应,包括光检测和 C-H 羟基化。虽然自由基基本步骤的力量是不可否认的,但对这些在催化中的基本理解和应用能力仍然不发达,这限制了化学家进行新反应的调色板。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号