当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Merging Organocatalysis with 1,2-Boronate Rearrangement: A Lewis Base-Catalyzed Asymmetric Multicomponent Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-24 , DOI: 10.1021/jacs.4c11113 Hong-Cheng Shen, Varinder K. Aggarwal
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-24 , DOI: 10.1021/jacs.4c11113 Hong-Cheng Shen, Varinder K. Aggarwal
|
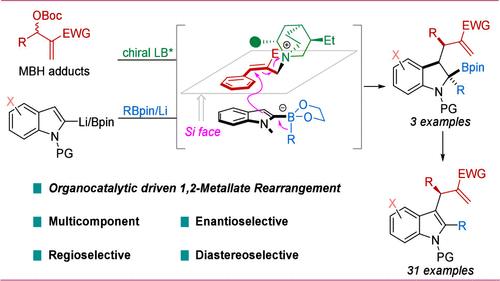
Catalytic asymmetric multicomponent 1,2-boronate rearrangements provide a practical approach for synthesizing highly valuable enantioenriched boronic esters. When applied to alkenyl or heteroaryl boronates, these reactions have relied mainly on transition-metal catalysis. Herein, we present an organocatalytic, Lewis base-catalyzed asymmetric multicomponent 1,2-boronate rearrangement, involving indoles, boronic esters, and Morita–Baylis–Hillman carbonates, leading to enantioenriched, highly substituted indole and indoline derivatives. Using cinchona alkaloid-based catalysts, high selectivity has been achieved, enabling expansion of the chemical space around pharmaceutically relevant indole and indoline derivatives.
中文翻译:

有机催化与 1,2-硼酸盐重排的合并:Lewis 碱催化的不对称多组分反应
催化不对称多组分 1,2-硼酸盐重排为合成高价值的对映体富集硼酯提供了一种实用方法。当应用于烯基或杂芳基硼酸盐时,这些反应主要依赖于过渡金属催化。在此,我们提出了一种有机催化的 Lewis 碱催化的不对称多组分 1,2-硼酸盐重排,涉及吲哚、硼酯和 Morita-Baylis-Hillman 碳酸盐,导致对映体富集、高度取代的吲哚和吲哚啉衍生物。使用基于金鸡纳生物碱的催化剂,已经实现了高选择性,从而能够扩大围绕制药相关吲哚和吲哚啉衍生物的化学空间。
更新日期:2024-09-24
中文翻译:

有机催化与 1,2-硼酸盐重排的合并:Lewis 碱催化的不对称多组分反应
催化不对称多组分 1,2-硼酸盐重排为合成高价值的对映体富集硼酯提供了一种实用方法。当应用于烯基或杂芳基硼酸盐时,这些反应主要依赖于过渡金属催化。在此,我们提出了一种有机催化的 Lewis 碱催化的不对称多组分 1,2-硼酸盐重排,涉及吲哚、硼酯和 Morita-Baylis-Hillman 碳酸盐,导致对映体富集、高度取代的吲哚和吲哚啉衍生物。使用基于金鸡纳生物碱的催化剂,已经实现了高选择性,从而能够扩大围绕制药相关吲哚和吲哚啉衍生物的化学空间。













































 京公网安备 11010802027423号
京公网安备 11010802027423号