当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Temperature on the Self-Assembly of Fibrinogen in Thrombin-Free Solutions
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-09-24 , DOI: 10.1021/acs.jpclett.4c02180 Leon Koch, Sanjib Saha, Klaus Huber
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-09-24 , DOI: 10.1021/acs.jpclett.4c02180 Leon Koch, Sanjib Saha, Klaus Huber
|
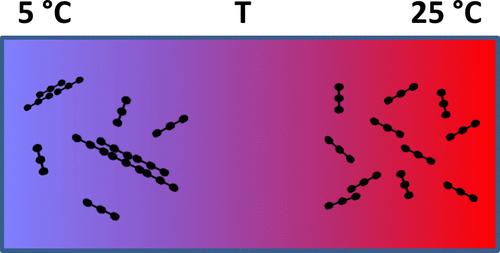
Self-assembly of thrombin-free solutions of fibrinogen can be triggered not only by a drop in the ionic strength but also by an appropriate decrease in temperature. Accordingly, an in situ study of self-assembly of fibrinogen in saline buffered solution is carried out by means of time-resolved light scattering providing the molar mass, geometric size, and hydrodynamic radius of the growing intermediates. The resulting data provide access to the morphology of the intermediates and to the mechanism in which these intermediates grow during the early stages of self-assembly. Modeling the results of concentration dependent experiments based on temperature gradients in terms of a chain growth mechanism leads to the corresponding molar standard enthalpy and entropy of aggregation.
中文翻译:

温度对无凝血酶溶液中纤维蛋白原自组装的影响
纤维蛋白原的无凝血酶溶液的自组装不仅可以通过离子强度的下降来触发,还可以通过温度的适当降低来触发。因此,通过时间分辨光散射对盐缓冲溶液中纤维蛋白原的自组装进行原位研究,提供生长中间体的摩尔质量、几何尺寸和流体动力学半径。由此产生的数据可以了解中间体的形态以及这些中间体在自组装早期阶段生长的机制。根据链增长机制对基于温度梯度的浓度依赖实验的结果进行建模,得出相应的摩尔标准聚集熵和熵。
更新日期:2024-09-24
中文翻译:

温度对无凝血酶溶液中纤维蛋白原自组装的影响
纤维蛋白原的无凝血酶溶液的自组装不仅可以通过离子强度的下降来触发,还可以通过温度的适当降低来触发。因此,通过时间分辨光散射对盐缓冲溶液中纤维蛋白原的自组装进行原位研究,提供生长中间体的摩尔质量、几何尺寸和流体动力学半径。由此产生的数据可以了解中间体的形态以及这些中间体在自组装早期阶段生长的机制。根据链增长机制对基于温度梯度的浓度依赖实验的结果进行建模,得出相应的摩尔标准聚集熵和熵。













































 京公网安备 11010802027423号
京公网安备 11010802027423号