Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interleukin-34-orchestrated tumor-associated macrophage reprogramming is required for tumor immune escape driven by p53 inactivation
Immunity ( IF 25.5 ) Pub Date : 2024-09-24 , DOI: 10.1016/j.immuni.2024.08.015 Zhigang Nian, Yingchao Dou, Yiqing Shen, Jintang Liu, Xianghui Du, Yong Jiang, Yonggang Zhou, Binqing Fu, Rui Sun, Xiaohu Zheng, Zhigang Tian, Haiming Wei
Immunity ( IF 25.5 ) Pub Date : 2024-09-24 , DOI: 10.1016/j.immuni.2024.08.015 Zhigang Nian, Yingchao Dou, Yiqing Shen, Jintang Liu, Xianghui Du, Yong Jiang, Yonggang Zhou, Binqing Fu, Rui Sun, Xiaohu Zheng, Zhigang Tian, Haiming Wei
|
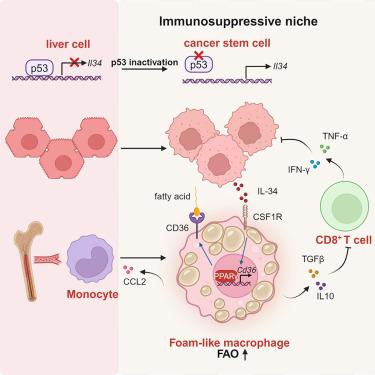
As the most frequent genetic alteration in cancer, more than half of human cancers have p53 mutations that cause transcriptional inactivation. However, how p53 modulates the immune landscape to create a niche for immune escape remains elusive. We found that cancer stem cells (CSCs) established an interleukin-34 (IL-34)-orchestrated niche to promote tumorigenesis in p53-inactivated liver cancer. Mechanistically, we discovered that Il34 is a gene transcriptionally repressed by p53, and p53 loss resulted in IL-34 secretion by CSCs. IL-34 induced CD36-mediated elevations in fatty acid oxidative metabolism to drive M2-like polarization of foam-like tumor-associated macrophages (TAMs). These IL-34-orchestrated TAMs suppressed CD8+ T cell-mediated antitumor immunity to promote immune escape. Blockade of the IL-34-CD36 axis elicited antitumor immunity and synergized with anti-PD-1 immunotherapy, leading to a complete response. Our findings reveal the underlying mechanism of p53 modulation of the tumor immune microenvironment and provide a potential target for immunotherapy of cancer with p53 inactivation.
中文翻译:

白细胞介素 34 协调的肿瘤相关巨噬细胞重编程是 p53 失活驱动的肿瘤免疫逃逸所必需的
作为癌症中最常见的基因改变,超过一半的人类癌症具有导致转录失活的 p53 突变。然而,p53 如何调节免疫景观以创造免疫逃逸的生态位仍然难以捉摸。我们发现癌症干细胞 (CSCs) 建立了白细胞介素 34 (IL-34) 编排的生态位,以促进 p53 灭活肝癌的肿瘤发生。从机制上讲,我们发现 Il34 是一个被 p53 转录抑制的基因,p53 缺失导致 CSCs 分泌 IL-34。IL-34 诱导 CD36 介导的脂肪酸氧化代谢升高,以驱动泡沫样肿瘤相关巨噬细胞 (TAM) 的 M2 样极化。这些 IL-34 编排的 TAMs 抑制 CD8+ T 细胞介导的抗肿瘤免疫,促进免疫逃逸。阻断 IL-34-CD36 轴引发抗肿瘤免疫,并与抗 PD-1 免疫疗法协同作用,导致完全反应。我们的研究结果揭示了 p53 调节肿瘤免疫微环境的潜在机制,并为 p53 失活癌症的免疫治疗提供了潜在的靶点。
更新日期:2024-09-24
中文翻译:

白细胞介素 34 协调的肿瘤相关巨噬细胞重编程是 p53 失活驱动的肿瘤免疫逃逸所必需的
作为癌症中最常见的基因改变,超过一半的人类癌症具有导致转录失活的 p53 突变。然而,p53 如何调节免疫景观以创造免疫逃逸的生态位仍然难以捉摸。我们发现癌症干细胞 (CSCs) 建立了白细胞介素 34 (IL-34) 编排的生态位,以促进 p53 灭活肝癌的肿瘤发生。从机制上讲,我们发现 Il34 是一个被 p53 转录抑制的基因,p53 缺失导致 CSCs 分泌 IL-34。IL-34 诱导 CD36 介导的脂肪酸氧化代谢升高,以驱动泡沫样肿瘤相关巨噬细胞 (TAM) 的 M2 样极化。这些 IL-34 编排的 TAMs 抑制 CD8+ T 细胞介导的抗肿瘤免疫,促进免疫逃逸。阻断 IL-34-CD36 轴引发抗肿瘤免疫,并与抗 PD-1 免疫疗法协同作用,导致完全反应。我们的研究结果揭示了 p53 调节肿瘤免疫微环境的潜在机制,并为 p53 失活癌症的免疫治疗提供了潜在的靶点。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号