当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Marmoset and human trophoblast stem cells differ in signaling requirements and recapitulate divergent modes of trophoblast invasion
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-09-24 , DOI: 10.1016/j.stem.2024.09.004 Dylan Siriwardena, Clara Munger, Christopher Penfold, Timo N. Kohler, Antonia Weberling, Madeleine Linneberg-Agerholm, Erin Slatery, Anna L. Ellermann, Sophie Bergmann, Stephen J. Clark, Thomas M. Rawlings, Joshua M. Brickman, Wolf Reik, Jan J. Brosens, Magdalena Zernicka-Goetz, Erika Sasaki, Rüdiger Behr, Florian Hollfelder, Thorsten E. Boroviak
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-09-24 , DOI: 10.1016/j.stem.2024.09.004 Dylan Siriwardena, Clara Munger, Christopher Penfold, Timo N. Kohler, Antonia Weberling, Madeleine Linneberg-Agerholm, Erin Slatery, Anna L. Ellermann, Sophie Bergmann, Stephen J. Clark, Thomas M. Rawlings, Joshua M. Brickman, Wolf Reik, Jan J. Brosens, Magdalena Zernicka-Goetz, Erika Sasaki, Rüdiger Behr, Florian Hollfelder, Thorsten E. Boroviak
|
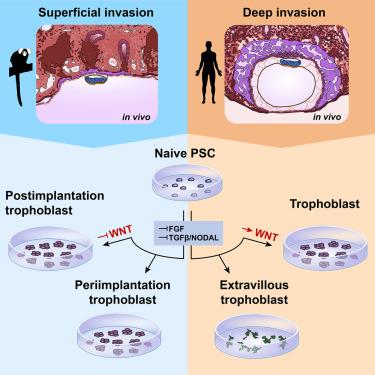
Early human trophoblast development has remained elusive due to the inaccessibility of the early conceptus. Non-human primate models recapitulate many features of human development and allow access to early postimplantation stages. Here, we tracked the pre- to postimplantation transition of the trophoblast lineage in superficially implanting marmoset embryos in vivo. We differentiated marmoset naive pluripotent stem cells into trophoblast stem cells (TSCs), which exhibited trophoblast-specific transcriptome, methylome, differentiation potential, and long-term self-renewal. Notably, human TSC culture conditions failed to support marmoset TSC derivation, instead inducing an extraembryonic mesoderm-like fate in marmoset cells. We show that combined MEK, TGF-β/NODAL, and histone deacetylase inhibition stabilizes a periimplantation trophoblast-like identity in marmoset TSCs. By contrast, these conditions differentiated human TSCs toward extravillous trophoblasts. Our work presents a paradigm to harness the evolutionary divergence in implantation strategies to elucidate human trophoblast development and invasion.
中文翻译:

狨猴和人类滋养层干细胞的信号传导要求不同,并概括了滋养层侵袭的不同模式
由于早期概念的不可及性,早期人类滋养层的发育仍然难以捉摸。非人灵长类动物模型概括了人类发育的许多特征,并允许进入植入后的早期阶段。在这里,我们追踪了在体内浅表植入狨猴胚胎 中滋养层谱系的植入前到植入后过渡。我们将狨猴幼稽多能干细胞分化为滋养层干细胞 (TSCs),其表现出滋养层特异性转录组、甲基化组、分化潜能和长期自我更新。值得注意的是,人类 TSC 培养条件不支持狨猴 TSC 衍生,而是在狨猴细胞中诱导胚外中胚层样命运。我们表明,MEK 、 TGF-β/NODAL 和组蛋白脱乙酰酶抑制联合稳定了狨猴 TSCs 中植入周围滋养层样身份。相比之下,这些条件将人类 TSC 区分为绒毛外滋养层细胞。我们的工作提出了一种范式,可以利用植入策略的进化分歧来阐明人类滋养层的发育和侵袭。
更新日期:2024-09-24
中文翻译:

狨猴和人类滋养层干细胞的信号传导要求不同,并概括了滋养层侵袭的不同模式
由于早期概念的不可及性,早期人类滋养层的发育仍然难以捉摸。非人灵长类动物模型概括了人类发育的许多特征,并允许进入植入后的早期阶段。在这里,我们追踪了在体内浅表植入狨猴胚胎 中滋养层谱系的植入前到植入后过渡。我们将狨猴幼稽多能干细胞分化为滋养层干细胞 (TSCs),其表现出滋养层特异性转录组、甲基化组、分化潜能和长期自我更新。值得注意的是,人类 TSC 培养条件不支持狨猴 TSC 衍生,而是在狨猴细胞中诱导胚外中胚层样命运。我们表明,MEK 、 TGF-β/NODAL 和组蛋白脱乙酰酶抑制联合稳定了狨猴 TSCs 中植入周围滋养层样身份。相比之下,这些条件将人类 TSC 区分为绒毛外滋养层细胞。我们的工作提出了一种范式,可以利用植入策略的进化分歧来阐明人类滋养层的发育和侵袭。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号