当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-cost dealkalization of bauxite residue by gypsum coordinated with industrial iron salt: Mineralogical mechanisms and field practice
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-09-21 , DOI: 10.1016/j.cej.2024.156022 Shiwei Huang, Yifan Jiang, Feng Zhu, Mingxing Zhu, Yufei Zhang, Xuanzhi Zhu, Ziying Zhang, Jun Jiang, Shengguo Xue
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-09-21 , DOI: 10.1016/j.cej.2024.156022 Shiwei Huang, Yifan Jiang, Feng Zhu, Mingxing Zhu, Yufei Zhang, Xuanzhi Zhu, Ziying Zhang, Jun Jiang, Shengguo Xue
|
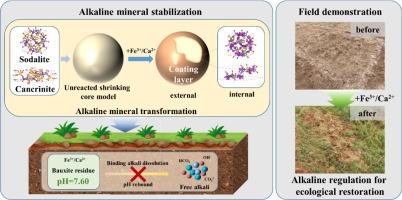
The sustained-release of alkalinity in bauxite residue hinders the soil formation and ecological restoration processes in disposal areas. Ferric salts (Fe3+), known for their rapid neutralization reaction process and straightforward operational simplicity, showed a synergistic advantage with gypsum (CaSO4) in the alkaline regulation of bauxite residue. Nevertheless, the mineralogical mechanisms of the reaction between Fe3+ and Ca2+ and alkaline minerals in bauxite residue remained unclear. The present study aimed to elucidate the geochemical stability mechanisms of sodalite and cancrinite, major alkaline minerals in bauxite residue, driven by Fe3+ and Ca2+ from a mineral structural perspective and the integrated restoration effect was demonstrated by the field experiment. Unreacted shrinking core model (USCM) calculations demonstrated that Fe3+ was able to enter the lattice structure of sodalite and cancrinite to replace Na+ and assisted Ca2+ in successfully replacing Na+ in cancrinite. Moreover, Fe3+ and Ca2+ facilitated the formation of insoluble coating layers on their surface. In the lattice structure of sodalite and cancrinite, the replacement reaction for Na+ was influenced by the competition between Fe3+ and Ca2+. The Ca2+ preferentially replaced Na+, while Fe3+ accumulated near the Si-O bonds, balancing the negative charges of the Si-O and Al-O tetrahedra structure and further inhibited alkaline mineral dissolution. The pH and EC of bauxite residue decreased from 10.52 to 7.60 and 712 μS/cm to 501 μS/cm, respectively, after a one-year field-scale amelioration by the integration of Fe3+ and Ca2+. These findings highlighted the synergistic roles of Fe3+ and Ca2+ in regulating the alkalinity of bauxite residue and provided a practical strategy for ecological restoration in disposal areas.
中文翻译:

石膏与工业铁盐配合对铝土矿残渣进行低成本脱碳:矿物学机制和现场实践
铝土矿残留物中碱度的持续释放阻碍了处置区的土壤形成和生态恢复过程。铁盐 (Fe3+) 以其快速的中和反应过程和简单的操作简单性而闻名,在铝土矿渣的碱性调节方面显示出与石膏 (CaSO4) 的协同优势。然而,铝土矿渣中 Fe3+ 和 Ca2+ 与碱性矿物反应的矿物学机制仍不清楚。本研究旨在从矿物结构角度阐明铝土矿渣中主要碱性矿物方钠石和坎克利石在 Fe3+ 和 Ca2+ 驱动的地球化学稳定性机制,并通过现场试验证明了其综合修复效果。未反应收缩芯模型 (USCM) 计算表明,Fe3+ 能够进入方钠石和坎克灵石的晶格结构以取代 Na+,并协助 Ca2+ 成功取代坎克灵石中的 Na+。此外,Fe3+ 和 Ca2+ 促进了其表面不溶性涂层的形成。在方钠石和钙镁石的晶格结构中,Na+ 的取代反应受到 Fe3+ 和 Ca2+ 之间竞争的影响。Ca2+ 优先取代 Na+,而 Fe3+ 积累在 Si-O 键附近,平衡了 Si-O 和 Al-O 四面体结构的负电荷,进一步抑制了碱性矿物的溶解。铝土矿残渣的 pH 和 EC 由 10.52 降低到 7。通过整合 Fe3+ 和 Ca2+ 进行为期一年的现场规模改善后,分别为 60 和 712 μS/cm 至 501 μS/cm。这些发现突出了 Fe3+ 和 Ca2+ 在调节铝土矿渣碱度中的协同作用,为处置区生态恢复提供了实用的策略。
更新日期:2024-09-21
中文翻译:

石膏与工业铁盐配合对铝土矿残渣进行低成本脱碳:矿物学机制和现场实践
铝土矿残留物中碱度的持续释放阻碍了处置区的土壤形成和生态恢复过程。铁盐 (Fe3+) 以其快速的中和反应过程和简单的操作简单性而闻名,在铝土矿渣的碱性调节方面显示出与石膏 (CaSO4) 的协同优势。然而,铝土矿渣中 Fe3+ 和 Ca2+ 与碱性矿物反应的矿物学机制仍不清楚。本研究旨在从矿物结构角度阐明铝土矿渣中主要碱性矿物方钠石和坎克利石在 Fe3+ 和 Ca2+ 驱动的地球化学稳定性机制,并通过现场试验证明了其综合修复效果。未反应收缩芯模型 (USCM) 计算表明,Fe3+ 能够进入方钠石和坎克灵石的晶格结构以取代 Na+,并协助 Ca2+ 成功取代坎克灵石中的 Na+。此外,Fe3+ 和 Ca2+ 促进了其表面不溶性涂层的形成。在方钠石和钙镁石的晶格结构中,Na+ 的取代反应受到 Fe3+ 和 Ca2+ 之间竞争的影响。Ca2+ 优先取代 Na+,而 Fe3+ 积累在 Si-O 键附近,平衡了 Si-O 和 Al-O 四面体结构的负电荷,进一步抑制了碱性矿物的溶解。铝土矿残渣的 pH 和 EC 由 10.52 降低到 7。通过整合 Fe3+ 和 Ca2+ 进行为期一年的现场规模改善后,分别为 60 和 712 μS/cm 至 501 μS/cm。这些发现突出了 Fe3+ 和 Ca2+ 在调节铝土矿渣碱度中的协同作用,为处置区生态恢复提供了实用的策略。













































 京公网安备 11010802027423号
京公网安备 11010802027423号