当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effects of primary cilia-mediated mechanical stimulation on nestin+-BMSCs during bone-tendon healing
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-09-19 , DOI: 10.1016/j.jare.2024.09.012 Huabin Chen, Han Xiao, Bing Wu, Xin Shi, Changbiao Guan, Jianzhong Hu, Tao Zhang, Hongbin Lu
中文翻译:

骨-肌腱愈合过程中原发性纤毛介导的机械刺激对巢蛋白+-BMSCs的影响
机械刺激已被证明可促进骨-肌腱界面 (BTI) 愈合,但机制仍不清楚。
探讨机械刺激对 BTI 愈合过程中巢蛋白 + 骨间充质干细胞 (BMSCs) 生物学行为的影响,并揭示机械刺激影响巢蛋白 + -BMSCs 上初级纤毛 BTI 愈合的机制。
构建在巢蛋白+-BMSCs条件敲除上具有原代纤毛的转基因示踪小鼠 (nestin creERT2:: IFT88fl/fl/ROSA26 YFP),并在巢蛋白 + -BMSCs 上具有正常纤毛的同窝小鼠 (nestin creERT2:: ROSA26 YFP) 为对照。建立小鼠冈上肌插入损伤模型后,在第 2 周 (每组 n = 5 )、第 4 周和第 8 周 (分别为每组 n = 15 个) 收集样本。在体内,通过组织学、影像学、生物力学和巢蛋白+-BMSCs的迁移评价修复效率,免疫荧光染色检测。在体外,通过拉力对巢蛋白 + BMSCs 进行分选和刺激,以研究初级纤毛介导的机械敏感基础的机制。
机械刺激 (MS) 加速了巢蛋白+-BMSCs 的募集,促进了成骨和软骨形成能力。组织学、影像学和生物力学结果显示,IFT88+/+, MS 组的 BTI 愈合质量优于其他组。在巢蛋白+-BMSCs中条件敲除 IFT88 后,BTI 的修复能力明显变差,即使机械刺激没有显着增加 (IFT88-/-,MS 组)。体外结果表明,拉伸载荷增强了具有正常纤毛的巢蛋白+-BMSCs 的增殖、迁移和成骨或成软骨基因的表达。另一方面,在抑制肌动蛋白 - Hippo/YAP 通路成分后,成骨和软骨生成表达显著降低。
初级纤毛介导的机械刺激通过肌动蛋白 Hippo/YAP 通路调节巢蛋白+-BMSCs 的成骨和成软骨分化潜能,进而促进 BTI 愈合过程。
更新日期:2024-09-19
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-09-19 , DOI: 10.1016/j.jare.2024.09.012 Huabin Chen, Han Xiao, Bing Wu, Xin Shi, Changbiao Guan, Jianzhong Hu, Tao Zhang, Hongbin Lu
|
Introduction
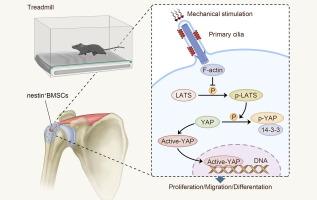
Mechanical stimulation has been proven to promote bone-tendon interface (BTI) healing, but the mechanism remains unclear.Objective
To investigate the effects of mechanical stimulation on the biological behavior of nestin+-bone mesenchymal stem cells (BMSCs) during the BTI healing, and to reveal the mechanisms of mechanical stimulation affecting BTI healing by primary cilia on the nestin+-BMSCs.Methods
Transgenic tracing mice (nestin creERT2:: IFT88fl/fl/ROSA26 YFP) with primary cilia on nestin+-BMSCs conditioned knocked out were constructed, and the littermates (nestin creERT2:: ROSA26 YFP) with normal cilia on nestin+-BMSCs were the control. After establishing mouse supraspinatus insertion injury models, samples were collected at week-2 (n = 5 per group), 4 and 8 (n = 15 per group, respectively). In vivo, the repair efficiency was evaluated by histology, imaging, biomechanics, and the migration of nestin+-BMSCs, detected by immunofluorescence staining. In vitro, nestin+ BMSCs were sorted and stimulated by tensile force to study the mechanisms of primary cilium-mediated mechanosensitive basis.Results
Mechanical stimulation (MS) accelerated the recruitment of nestin+-BMSCs and promoted osteogenic and chondrogenic capacity. Histological, imaging and biomechanical results showed that the BTI healing quality of the IFT88+/+, MS group was better than that of the other groups. After the conditionally knockout IFT88 in nestin+-BMSCs, the repair ability of the BTI was obviously deteriorated, even though mechanical stimulation did not increase significantly (IFT88-/-, MS group). In vitro results showed the tensile loading enhanced the proliferation, migration and osteogenic or chondrogenic gene expression of nestin+-BMSCs with normal cilia. On the other hand, osteogenesis and chondrogenic expression were significantly decreased after inhibiting actin- Hippo/YAP pathway components.Conclusion
The primary cilia mediated mechanical stimulation regulated osteogenic and chondrogenic differentiation potential of nestin+-BMSCs through the actin- Hippo/YAP pathway, and then promoted the BTI healing process.中文翻译:

骨-肌腱愈合过程中原发性纤毛介导的机械刺激对巢蛋白+-BMSCs的影响
介绍
机械刺激已被证明可促进骨-肌腱界面 (BTI) 愈合,但机制仍不清楚。
目的
探讨机械刺激对 BTI 愈合过程中巢蛋白 + 骨间充质干细胞 (BMSCs) 生物学行为的影响,并揭示机械刺激影响巢蛋白 + -BMSCs 上初级纤毛 BTI 愈合的机制。
方法
构建在巢蛋白+-BMSCs条件敲除上具有原代纤毛的转基因示踪小鼠 (nestin creERT2:: IFT88fl/fl/ROSA26 YFP),并在巢蛋白 + -BMSCs 上具有正常纤毛的同窝小鼠 (nestin creERT2:: ROSA26 YFP) 为对照。建立小鼠冈上肌插入损伤模型后,在第 2 周 (每组 n = 5 )、第 4 周和第 8 周 (分别为每组 n = 15 个) 收集样本。在体内,通过组织学、影像学、生物力学和巢蛋白+-BMSCs的迁移评价修复效率,免疫荧光染色检测。在体外,通过拉力对巢蛋白 + BMSCs 进行分选和刺激,以研究初级纤毛介导的机械敏感基础的机制。
结果
机械刺激 (MS) 加速了巢蛋白+-BMSCs 的募集,促进了成骨和软骨形成能力。组织学、影像学和生物力学结果显示,IFT88+/+, MS 组的 BTI 愈合质量优于其他组。在巢蛋白+-BMSCs中条件敲除 IFT88 后,BTI 的修复能力明显变差,即使机械刺激没有显着增加 (IFT88-/-,MS 组)。体外结果表明,拉伸载荷增强了具有正常纤毛的巢蛋白+-BMSCs 的增殖、迁移和成骨或成软骨基因的表达。另一方面,在抑制肌动蛋白 - Hippo/YAP 通路成分后,成骨和软骨生成表达显著降低。
结论
初级纤毛介导的机械刺激通过肌动蛋白 Hippo/YAP 通路调节巢蛋白+-BMSCs 的成骨和成软骨分化潜能,进而促进 BTI 愈合过程。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号