当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent synthetic strategies for the functionalization of fused bicyclic heteroaromatics using organo-Li, -Mg and -Zn reagents
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-09-23 , DOI: 10.1039/d4cs00369a Vasudevan Dhayalan, Vishal S. Dodke, Marappan Pradeep Kumar, Hatice Seher Korkmaz, Anja Hoffmann-Röder, Pitchamuthu Amaladass, Rambabu Dandela, Ragupathy Dhanusuraman, Paul Knochel
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2024-09-23 , DOI: 10.1039/d4cs00369a Vasudevan Dhayalan, Vishal S. Dodke, Marappan Pradeep Kumar, Hatice Seher Korkmaz, Anja Hoffmann-Röder, Pitchamuthu Amaladass, Rambabu Dandela, Ragupathy Dhanusuraman, Paul Knochel
|
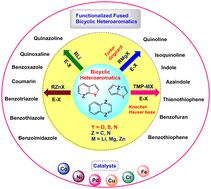
This review highlights the use of functionalized organo-Li, -Mg and -Zn reagents for the construction and selective functionalization of 5- and 6-membered fused bicyclic heteroaromatics. Special attention is given to the discussion of advanced syntheses for the preparation of highly functionalized heteroaromatic scaffolds, including quinolines, naphthyridines, indoles, benzofurans, benzothiophenes, benzoxazoles, benzothiazoles, benzopyrimidines, anthranils, thienothiophenes, purine coumarins, chromones, quinolones and phthalazines and their fused heterocyclic derivatives. The organometallic reagents used for the desired functionalizations of these scaffolds are generally prepared in situ using the following methods: (i) through directed selective metalation reactions (DoM), (ii) by means of halogen/metal exchange reactions, (iii) through oxidative metal insertions (Li, Mg, Zn), and (iv) by transmetalation reactions (organo-Li and Mg transmetalations with ZnCl2 or ZnO(Piv)2). The resulting reactive organometallic reagents allow a wide range of C–C, C–N and C–X cross-coupling reactions with different electrophiles, employing in particular Kumada or Negishi protocols among other transition metal (Pd, Ni, Co, Cu, Cr, Fe, etc.)-catalyzed processes. In addition, key developments concerning selective metalation techniques will be presented, which rely on the use of RLi, LDA and TMP metal bases. These methods are now widely employed in organic synthetic chemistry and have proven to be particularly valuable for drug development programs in the pharmaceutical industry. New and improved protocols have resulted in many Li, Mg and Zn organyls now being compatible with functionalized aryl, heteroaryl, alkenyl, alkynyl and alkyl compounds even in the presence of labile functional groups, making these reagents well-suited for C(sp2)–C(sp2), C(sp2)–C(sp) and C(sp2)–C(sp3) cross-coupling reactions with fused heteroaryl halides. In addition, the use of some transition metal-catalyzed processes occasionally allows a reversed role of the reactants in cross-coupling reactions, providing alternative synthetic routes for the preparation of fused heteroaromatic-based bioactive drugs and natural products. In line with this, this article points to novel methods for the functionalization of bicyclic heteroaromatic scaffolds by organometallic reagents that have been published in the period 2010–2023.
中文翻译:

使用 organo-Li、-Mg 和 -Zn 试剂对熔融双环杂芳烃进行官能化的最新合成策略
本文重点介绍了功能化有机 Li、-Mg 和 -Zn 试剂在 5 元和 6 元熔融双环杂芳烃的构建和选择性功能化中的应用。特别关注了用于制备高度功能化杂芳烃支架的高级合成的讨论,包括喹啉、萘啶、吲哚、苯并呋喃、苯并噻吩、苯并噻唑、苯并噻唑、苯并嘧啶、蒽、噻噻吩、嘌呤香豆素、色酮、喹诺酮类和邻苯二甲酸酯及其稠合杂环衍生物。用于这些支架所需功能化的有机金属试剂通常使用以下方法原位制备:(i) 通过定向选择性金属化反应 (DoM),(ii) 通过卤素/金属交换反应,(iii) 通过氧化金属插入物(Li、Mg、Zn),以及 (iv) 通过金属转移反应(有机 Li 和 Mg 与 ZnCl2 或 ZnO (Piv) 的金属转移2).所得的反应性有机金属试剂允许与不同的亲电试剂进行广泛的 C-C、C-N 和 C-X 交叉偶联反应,特别是采用 Kumada 或 Negishi 方案以及其他过渡金属(Pd、Ni、Co、Cu、Cr、Fe 等)催化过程。此外,还将介绍有关选择性金属化技术的关键发展,这些技术依赖于 RLi、LDA 和 TMP 金属基的使用。这些方法现在广泛用于有机合成化学,并已被证明对制药行业的药物开发计划特别有价值。 新的和改进的方案使许多 Li、Mg 和 Zn 有机基现在与官能化芳基、杂芳基、烯基、炔基和烷基化合物兼容,即使在存在不稳定官能团的情况下也是如此,使这些试剂非常适合 C(sp2)–C(sp2)、C(sp2)–C(sp) 和 C(sp2)–C(sp3) 与稠合杂芳基卤化物的交叉偶联反应。此外,使用一些过渡金属催化过程偶尔会允许反应物在交叉偶联反应中发挥相反的作用,为制备基于熔融杂芳烃的生物活性药物和天然产物提供替代合成路线。与此一致,本文指出了 2010-2023 年期间发表的有机金属试剂对双环杂芳烃支架进行官能化的新方法。
更新日期:2024-09-24
中文翻译:

使用 organo-Li、-Mg 和 -Zn 试剂对熔融双环杂芳烃进行官能化的最新合成策略
本文重点介绍了功能化有机 Li、-Mg 和 -Zn 试剂在 5 元和 6 元熔融双环杂芳烃的构建和选择性功能化中的应用。特别关注了用于制备高度功能化杂芳烃支架的高级合成的讨论,包括喹啉、萘啶、吲哚、苯并呋喃、苯并噻吩、苯并噻唑、苯并噻唑、苯并嘧啶、蒽、噻噻吩、嘌呤香豆素、色酮、喹诺酮类和邻苯二甲酸酯及其稠合杂环衍生物。用于这些支架所需功能化的有机金属试剂通常使用以下方法原位制备:(i) 通过定向选择性金属化反应 (DoM),(ii) 通过卤素/金属交换反应,(iii) 通过氧化金属插入物(Li、Mg、Zn),以及 (iv) 通过金属转移反应(有机 Li 和 Mg 与 ZnCl2 或 ZnO (Piv) 的金属转移2).所得的反应性有机金属试剂允许与不同的亲电试剂进行广泛的 C-C、C-N 和 C-X 交叉偶联反应,特别是采用 Kumada 或 Negishi 方案以及其他过渡金属(Pd、Ni、Co、Cu、Cr、Fe 等)催化过程。此外,还将介绍有关选择性金属化技术的关键发展,这些技术依赖于 RLi、LDA 和 TMP 金属基的使用。这些方法现在广泛用于有机合成化学,并已被证明对制药行业的药物开发计划特别有价值。 新的和改进的方案使许多 Li、Mg 和 Zn 有机基现在与官能化芳基、杂芳基、烯基、炔基和烷基化合物兼容,即使在存在不稳定官能团的情况下也是如此,使这些试剂非常适合 C(sp2)–C(sp2)、C(sp2)–C(sp) 和 C(sp2)–C(sp3) 与稠合杂芳基卤化物的交叉偶联反应。此外,使用一些过渡金属催化过程偶尔会允许反应物在交叉偶联反应中发挥相反的作用,为制备基于熔融杂芳烃的生物活性药物和天然产物提供替代合成路线。与此一致,本文指出了 2010-2023 年期间发表的有机金属试剂对双环杂芳烃支架进行官能化的新方法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号