当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile heterolytic bond splitting of molecular chlorine upon reactions with Lewis bases: Comparison with ICl and I2
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-23 , DOI: 10.1002/jcc.27507 Anna V. Pomogaeva, Anna S. Lisovenko, Alexey Y. Timoshkin
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-23 , DOI: 10.1002/jcc.27507 Anna V. Pomogaeva, Anna S. Lisovenko, Alexey Y. Timoshkin
|
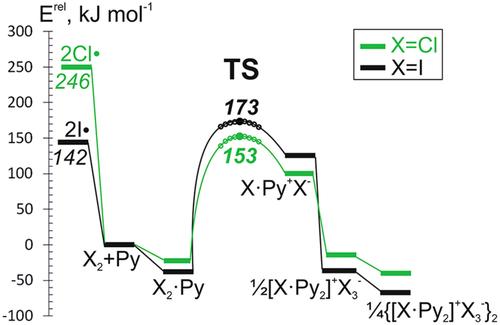
Formation of molecular complexes and subsequent heterolytic halogen-halogen bond splitting upon reactions of molecular Cl2 with nitrogen-containing Lewis bases (LB) are computationally studied at M06-2X/def2-TZVPD and for selected compounds at CCSD(T)/aug-cc-pvtz//CCSD/aug-cc-pvtz levels of theory. Obtained results are compared with data for ICl and I2 molecules. Reaction pathways indicate, that in case of Cl2∙LB complexes the activation energies for the heterolytic Cl-Cl bond splitting are lower than the activation energies of the homolytic splitting of Cl2 molecule into chlorine radicals. The heterolytic halogen splitting of molecular complexes of X2∙Py with formation of [XPy2]+…
中文翻译:

与 Lewis 碱反应时分子氯的易异解键分裂:与 ICl 和 I2 的比较
在 M06-2X/def2-TZVPD 和在 CCSD(T)/aug-cc-pvtz//CCSD/aug-cc-pvtz 理论水平上对选定化合物反应时分子复合物的形成和随后的异解卤素-卤素键分裂进行了计算研究。将获得的结果与 ICl 和 I2 分子的数据进行比较。反应途径表明,在 Cl2∙LB 络合物的情况下,异质溶解 Cl-Cl 键分裂的活化能低于 Cl2 分子均解分裂成氯自由基的活化能。X2∙Py 分子复合物的异质溶解卤素分裂,形成 [XPy2]+...气相中的 接触离子对在 Cl 2 和 I 2 的情况下略微吸热,但在 ICl 的情况下略微放热。 {[ClPy2]+... 的形成
更新日期:2024-09-23
中文翻译:

与 Lewis 碱反应时分子氯的易异解键分裂:与 ICl 和 I2 的比较
在 M06-2X/def2-TZVPD 和在 CCSD(T)/aug-cc-pvtz//CCSD/aug-cc-pvtz 理论水平上对选定化合物反应时分子复合物的形成和随后的异解卤素-卤素键分裂进行了计算研究。将获得的结果与 ICl 和 I2 分子的数据进行比较。反应途径表明,在 Cl2∙LB 络合物的情况下,异质溶解 Cl-Cl 键分裂的活化能低于 Cl2 分子均解分裂成氯自由基的活化能。X2∙Py 分子复合物的异质溶解卤素分裂,形成 [XPy2]+...气相中的































 京公网安备 11010802027423号
京公网安备 11010802027423号